- Submissions

Full Text
Journal of Biotechnology & Bioresearch
Phytochemical Screening and Antibacterial Activity of Vitex doniana Stem Bark Extracts Against Staphylococcus aureus and Pseudomonas aeruginosa Isolated from Infected Wound
Gambo SB1, Shehu AA2, Labaran HB1 and Ali M3*
1 Department of Biological Sciences, Rabiu Musa Kwankwaso College of Advanced and Remedial Studies, Nigeria
2 Department of Microbiology, Kano University of Science and Technology Wudil, Nigeria
3 Department of Biological Science, Federal University Gusau, Nigeria
*Corresponding author:Muhammad Ali, Department of Biological Science, Federal University Gusau, Nigeria
Submission: September 28, 2018;Published: October 16, 2018

Volume1 Issue3October 2018
Abstract
Wound infections have been recognized as the most critical problem especially in the presence of foreign materials that increase the risk of serious infection even with relatively small bacterial infection. This study was carried out to determine the antibacterial activity of stem bark extract of Vitex doniana against clinical isolates of Staphylococcus aureus and Pseudomonas aeruginosa obtained from infected wounds. Different concentrations of honey extract stem bark extracts of Vitex doniana (25, 50, 75 and 100mg/ml) were tested using agar well diffusion method to determine their antibacterial activity against S. aureus and P. aeruginosa isolated from infected wounds of patients attending Muhammad Abdullahi Wase Specialist Hospital, Kano. The phytochemical screening of the stem bark showed the presence of alkaloid, tannins, saponin, flavonoid, Anthraquinone, terpenoid, phenol and glycoside. The antibacterial activity of the stem bark extracts showed that the extracts were active against the isolates with higher antibacterial activity recorded in methanol extract compared to aqueous extract. The minimum inhibitory concentration (MIC) of the extract from this study ranges from 12.5-50mg/ml while the minimum bactericidal concentration (MBC) of the extracts ranges from 25-50mg/ml. Statistical analysis of the results showed that there is no significant different in the activity of the plant extracts at p< 0.05. Based on the results of this study, it is concluded that extracts are active against wound bacterial isolates and can be used as a therapy for wound infection.
keywords: Antibacterial activity; Pseudomonas aeruginosa; Phytochemicals; Staphylococcus aureus; Wound
Introduction
Wound infection can best describe as the deposition and multiplication of microbes especially bacteria in tissue with an associated host reaction [1]. The infection implicates the protective functions of skin, loss of continuity of epithelium tissue with or without loss of underlying connective tissue [2]. Wound infection can accidental, pathological or post-operative break down. However, the infection may be characterized by certain classic signs of pain, redness, swelling and fever [3,4]. Wound infection causes great distress in terms of associated mortality and morbidity, increased length of hospital stays, profound discomfort and significant increase in healthcare cost. Infection in a wound delays healing and may cause wound break down, herniation of the wound and complete wound dehiscence. Therefore, the knowledge of the causative agents of wound infection will assist in the control and prevention of such infection and also help in selecting empirical antimicrobial therapy for the control of causative microorganisms [4]. Some aerobic pathogenic organisms such P. aeruginosa, S. aureus and beta haemolytic Streptococci have been most frequently reported as the cause of delay wound healing [4,5]. A study on aerobic bacterial profile and antimicrobial susceptibility pattern of wound isolates in a South Indian tertiary care hospital revealed S. aureus (24.29%) was the most common isolate; this is followed by P. aeruginosa (21.49%), E. coli (14.02%), Klebsiella pneumoniae (12.15%), Streptococcus pyogenes (11.23%), S. epidermidis (09.35%) and Proteus sp (07.47%) [6]. In another study conducted by Verma and Chitra [7] on isolation of different types of bacteria from infected wound also revealed that Staphylococcus aureus as the most predominant microorganism accounted for 40% of the total isolates obtained, followed by Klebsiella sp (33%), P. aeruginosa (18%), E. coli (16%) and Proteus sp (07%) [7].
The emergence of bacterial antimicrobial resistance has made the choice of empirical therapy more difficult and expensive [8]. As result, regular screening of causative organism and determination of susceptibility pattern of such organisms to commonly used antibiotics is needed for empirical treatment of infections. Since ancient times, herbs and their essential oils have been known for their varying degrees of antimicrobial activity [9]. More recently, medicinal plant extracts were developed and proposed for use in food as natural antimicrobials [10]. Black-plum (V. doniana) of the family Verbanaceae is a tree crop that grows in open woodland and savannah regions of tropical Africa; it is the commonest of the Vitex species in West Africa [11].
The use of V. doniana suggests that it may possess antimicrobial activity. Various parts of the plant are used by traditional medicine practitioners in Nigeria in the management and treatment of several disorders which include rheumatism, hypertension, cancer, and inflammatory diseases [12]. Kilani [13] assess the antibacterial effect of whole stem bark of V. doniana against some Enterobacteriaceae and supports the use of V. doniana by traditional medicine practitioners in the treatment of dysentery and gastroenteritis. He stressed further that, antimicrobial activity of the V. doniana extract could be attributed to the presence of phenolic compounds that have been linked with antimicrobial properties [14]. Earlier workers have reported the use of the fruits and leaves and stem bark for medicinal purposes [12]. In Nigeria, from information available from the indigenous traditional healers, a decoction of the chopped stem bark part of V. doniana is prepared and taken orally for treatment of gastroenteritis and wound infection [12,15]. The aim of the study was to determine the antibacterial activity of V. doniana stem bark extract against S. aureus and P. aeruginosa isolated from infected wound of patients attending Muhammad Abdullahi Wase Specialist Hospital Kano
Materials and Methods
Sample collection
The study area is Kano metropolis, samples from infected wound patients were collected from Muhammad Abdullahi Wase Hospital in the state capital. Ethical approval (Issue number: HMB/ GEN/488/Vol. I) was obtained from Health Service Management board (HSMB) Kano State based on the consent of Muhammad Abdullahi Wase Specialists Hospital (MAWSH) Ethical Committees. The swap sticks containing a total of 52 samples from infected wound were collected from Microbiology laboratory of Abdullahi Wase Special Hospital Kano State for isolation and characterization of S. aureus and P. aeruginosa.
Isolation and identification of test organisms
The samples from swap containing pus from patients were inoculated onto the surface of nutrient agar plate and incubated for 24 hours at 37 °C. Each colony was isolated in a pure form by sub culturing for further studies and identification [16]. Distinctive morphological properties of each pure culture such as colony form, elevation of colony and colony margin were observed. Further microbial identification (Gram staining and biochemical characterization including catalase, coagulase and oxidase tests) was based on the methods of Holt et al. [17] & Sherman [18].
Gram staining
A drop of normal saline was placed on a well labeled clean grease-free glass slide using a sterile inoculating loop; a colony of an overnight culture of the bacterial isolate was emulsified with the normal saline to make a thin smear. The smear was air dried and then heat fixed. The slide was flooded with crystal violet (primary stain) for 30 seconds after which the stain was rinsed from the slide with water. The smear was flooded with Lugol’s iodine (mordant) to fix the primary stain. The iodine was rinsed with water after 60 seconds. The slide was then flooded with a decolorizer (acetone) and rinsed off almost immediately. The counter stain; safranin was added and left for 30 seconds before being rinsed off. The stained smear was air dried, and then observed under the microscope using X100 oil immersion objective lens of the microscope.
Catalase test
A microscope slide was placed inside a Petri dish. The Petri dish cover was kept available. Using sterile inoculating loop, a small amount of organism was collected from a well-isolated 24-hour colony and placed it onto the microscope slide. Using a dropper, 1 drop of 3% H2O2 was dropped onto the organism on the microscope slide.
Coagulase test
0.5ml of blood plasma was added into a test tube and 0.1ml of the test organism was added and observed after 30 minutes at room temperature
Oxidase test
A small piece of filter paper was soaked in 1% oxidase reagent and let dried. Using a sterile loop a well-isolated colony from a fresh 24-hours culture of bacterial plate was removed and rubbed onto treated filter paper and observed for color changes.
Collection and identification of plant material
The stem bark of V. doniana was collected at about 7:30 am on 17th November 2016 at Karfi village, Kura Local Government Area in Kano state, Nigeria. The identification and authentication of the plant materials was done at Herbarium in the department of Plant Science, Bayero University Kano with the following voucher number BUKHAN 0170, voucher specimens were deposited there for future reference. The part collected was washed thoroughly with distilled water and air-dried in a shade for two weeks, then cut into pieces and grinded into powder using a sterile pestle and mortar under laboratory condition. The powder was then kept in air tight container for future use.
Extraction of stem bark extract
Aqueous and methanol solvents were used for extraction process of the phytochemical components of the stem bark. For aqueous extract, water extraction method as described by Ahmed & Beg [19] was employed. During the process, 100g of the grounded stem bark was weighted and mixed with 500ml of distilled water in a sterile conical flask and kept for 4 days with intermittent shaking. The extracts were filtered using Whatman filter paper and the filtrates were concentrated in water bath at 50 °C. For ethanol, 100g of the powdered stem bark was extracted in 500ml of methanol for 3 days. The mixture was filtered using Whatman No.1 filter paper and the extract was evaporated to dryness using rotary evaporator at 40 °C. The residue obtained were diluted using 10% Dimethylsulphoxide (DMSO) to produce 100mg/ml of the extracts from which various concentration of 75, 50 and 25mg/ml were produced.
Phytochemical screening of the extracts
Phytochemical screening was conducted using laboratory method as described by Soforowa [12]. This was done to determine the presence of alkaloid, saponin, steroid, glycoside, tannin, terpenoid, anthraquinone, flavonoid and reducing sugar in the aqueous and ethanol extracts of the stem bark.
Test for alkaloids
Wagner’s test: To 0. lml of the extract in a test tube, 3 drops of Wagner’s reagent (Iodine in Potassium iodide) was added. Formation of brown/ reddish precipitate indicates the presence of Alkaloids.
Test for flavonoids
Lead acetate test: Extracts were treated with few drops of lead acetate solution. The formation of yellow colored precipitate indicates the presence of flavonoids.
Test for glycosides
Ten (10) ml of 50% Tetraoxosulphate (VI) acid was added to 1ml of the extract in a separate test tube and the mixture was heated gently for 15 minutes followed by addition of 10ml of Fehling solution and boiling. A brick red precipitate indicated the presence of glycosides.
Test for reducing sugar
Fehling’s test: To 1ml of the extract in a separate test tube, 2ml of distilled water was added, followed by addition of Fehlings solution (A+B) and the mixture was warmed at 40C. The appearance of brick red precipitate at the bottom of the test tube indicated the presence of reducing sugar.
Test for saponins
Foam test: Half gram (0.5g) of the powdered sample was dispensed in a test tube and 5ml of distilled water was added and shaken vigorously. Persistent froth (foam) that lasted for about 10 minutes indicated the presence of saponin.
Test for steroids
To 2ml of the sample, 2ml of acetic acid was added and the solution was kept under ice for cooling for few minutes. Then 2ml of concentrated Tetraoxosulphate (VI) acid was added carefully. Color changes, from violet to blue/bluish green indicated the presence of steroids.
Test for tannin
Gelatin test: To 2ml of the extract, 1% gelatin solution containing sodium chloride was added. The formation of white precipitate indicated the presence of tannins.
Test for phenol
Ferric chloride test: Extracts were treated with 5 drops of ferric chloride solution. The formation of bluish black color indicated the presence of phenols.
Test for terpenoid
Salkowski test: About 5ml of extract was added with 2ml of chloroform and 3ml of concentrated Tetraoxosulphate (VI) acid. Reddish brown colour at the interface indicates the presence of terpenoids.
Test for Anthraquinones
About 2ml of extract was added into a test tube, 5ml of benzene was added and shaken, then 5ml of 10% Ammonia solution was also added followed by shaking. The formation of pink/red/violet color in the lower phase is positive for Anthraquinone. Antibacterial activity of the extracts Agar well diffusion method was adapted to determine the antibacterial activity of the stem bark extracts against the test isolates in this study. During the process, 0.1ml of standardize organism (0.5 MacFarland standard) were introduced onto the surface of Mueller Hinton agar in a sterile Petri dish and labeled accordingly. A sterile cork borer 5mm was used to produce five wells at equal distance in the inoculated agar. The wells were filled with different concentrations of the extracts accordingly as 25, 50, 75 and 100mg/l while the last well contain 50mg/ml of Ciprofloxacin (Micro lab limited) which was used as positive control in the study. The agar plates were allowed to diffuse for a period of hour and incubated at 37 °C for 24 hours. After then, the diameter of the zones of inhibition around each well was measured to the nearest millimetres [20].
Determination of minimum inhibitory concentration (MIC) of the extracts
The MIC of the extracts was determined using broth dilution technique. Two-fold serial dilutions of the extracts were prepared by adding 2ml of 100mg/ml of the extract into a test tube containing 2ml of Nutrient broth, thus producing solution containing 50mg/ ml of the extract. The process continues serially up to test tube No. 5, hence producing the following concentrations; 50, 25, 12.5, 6.25 3.125mg/ml. Test tube No. 6 do not contain extracts and serve as negative control. Exactly 0.5 ml of 0.5 McFarland equivalent standards of test organisms were introduced into the test tubes and incubated at 37 °C for 24 hours. After incubation the test tubes were observed for growth by checking for turbidity [19].
Determination of minimum bactericidal concentration (MBC) of the extracts
From the result of MIC, the test tubes that did not show visible growth were used for MBC determination. About 0.1ml was aseptically transferred onto the surface of Mueller Hinton agar plates. The plates were incubated at 37 °C for 24 hours. The MBC of the extracts was recorded as the lowest concentration of the extract that had less than 99% growth on Mueller Hinton agar plates [19].
Statistical analysis
The data of average zone of inhibition produced by the isolates against the extracts used was analyzed using one-way Analysis of Variance (ANOVA) with the aid of statistical program SPSS (Statistical package for Social Sciences) version 21.0. Significance level for the differences was set at p< 0.05.
Results
Isolation and identification of test organisms
The result of identification of the test isolates is represented in Table 1. The tests conducted include Gram staining, Catalase test, Coagulase test, Oxidase test and Mannitol salt agar test for S. aureus. The S. aureus was found to be positive for Catalase and coagulase test but negative for oxidase test. On the other hand P. aeruginosa was positive for catalase and oxidase tests but negative for coagulase test.
Table 1:Gram staining and Biochemical characterization of the isolates.
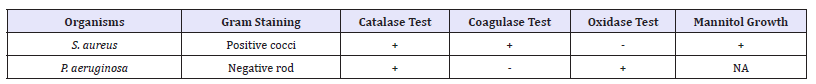
Key: +: Positive, -: Negative, NA: Not Applicable
Phytochemical screening
Table 2:Phytochemical Constituent of Stem Bark Extract of Vitex doniana.
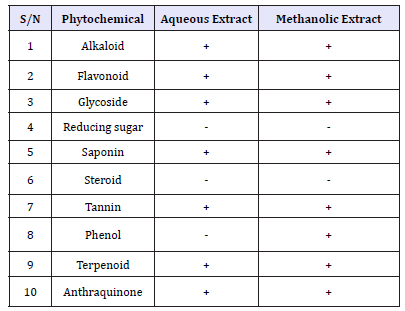
Key: + = Presence of Phytochemical. - = Absent of Phytochemical
The phytochemical constituents of aqueous and methanolic stem bark extract of V. doniana are presented in Table 2. The result showed that the aqueous and methanolic stem bark extracts contained the following Phytochemicals alkaloid, saponin, tannin, Anthraquinone, Flavonoid, phenols, terpenoid and glycoside. On the other hand steroid and reducing sugar were absent.
Antibacterial Activity
Aqueous extracts
The antibacterial activity of different concentration of V. doniana aqueous stem bark extract against S. aureus recovered from different wound samples is presented in Table 3. The result showed that higher zone of inhibition is shown by isolate S3 17.3mm at concentration of 100mg/ml. Isolate S1 was found to be more resistant to the extracts. Zones of inhibition shown recorded by the control ranges from 15-22mm. The antibacterial activity of different concentration of V. doniana aqueous stem bark extract against P. aeruginosa recovered from different wound samples is presented in Table 4. The result showed that higher zone of inhibition is shown by isolate P3 16.6mm at concentration of 100mg/ml. No zone of inhibition was found in P2 and P4 Zones of inhibition recorded by the control ranged from 17-19mm.
Table 3:Antibacterial Activity of V. doniana aqueous stem bark extract against S. aureus recovered from different wound samples with standard error.
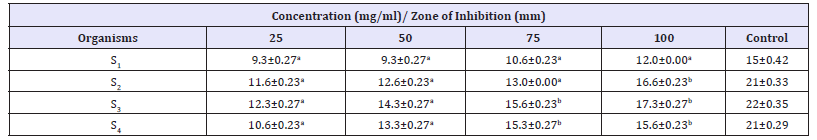
Key: Values having different superscript in the same row are considered significantly different at probability level
of p< 0.05. S1= S. aureus recovered from trauma wound, S2= S. aureus recovered from surgical wound, S3= S. aureus
recovered from sepsis wound, S4= S. aureus recovered from bite wound, Control=Ciprofloxacin (50mg/ml).
Table 4:Antibacterial Activity of V. doniana aqueous stem bark extract against P. aeruginosa recovered from different wound samples with standard error.
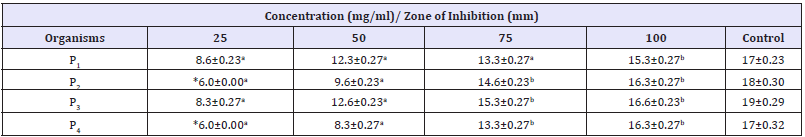
Key: Values having different superscript in the same row are considered significantly different at probability level of p< 0.05. P1= P. aeruginosa recovered from trauma wound, P2= P. aeruginosa recovered from surgical wound, P3= P. aeruginosa recovered from sepsis wound, P4= P. aeruginosa recovered from bite wound. *= No zone of inhibition, Control=Ciprofloxacin (50mg/ml)
Methanol extracts
The antibacterial activity of different concentration of V. doniana methanolic stem bark extract against S. aureus recovered from different wound samples is presented in Table 5. The result showed that higher zone of inhibition was shown by isolate S2 18.6mm at concentration of 100mg/ml. Zones of inhibition shown by the control ranged from 15-22mm. The antibacterial activity of different concentration of V. doniana methanolic stem bark extract against P. aeruginosa recovered from different wound samples is presented in Table 6. The result showed that higher zone of inhibition was shown by isolate P4 17.6 at concentration of 100mg/ ml. Zones of inhibition shown by the control ranged from 17-19mm.
Table 5:Antibacterial Activity of V. doniana methanolic stem bark extract against S. aureus recovered from different wound samples with standard error.
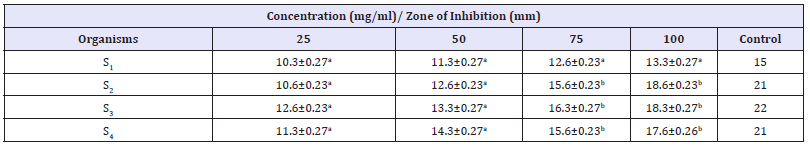
Key: Values having different superscript in the same row are considered significantly different at probability level of p< 0.05. The antibacterial activity of different concentration of V. doniana methanolic stem bark extract against P. aeruginosa recovered from different wound samples is presented in Table 6. The result showed that higher zone of inhibition was shown by isolate P4 17.6 at concentration of 100mg/ml. Zones of inhibition shown by the control ranged from 17-19mm.
Table 6:Antibacterial Activity of V. doniana methanolic stem bark extract against P. aeruginosa recovered from different wound samples with standard error.
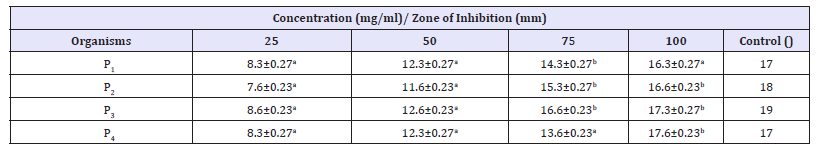
Key: Values having different superscript in the same row are considered significantly different at probability level of p< 0.05.
MIC and MBC of the extract
Table 7:
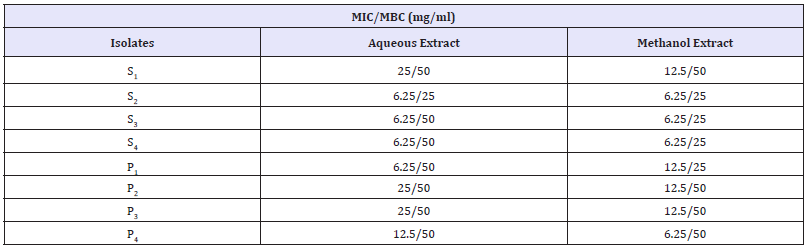
The minimum inhibitory concentration of aqueous and methanol stem bark extract is represented in Table 7 which showed dilutions of various concentrations of the extracts against the test organisms. Lower MIC was recorded in isolate S2, S3, S4 and P1 (12.5mg/ml) while highest MIC value was recorded in isolate S1, P2, and P3 (25 and 50mg/ml for methanol and aqueous extract respectively). The MBC of the plant extracts showed that the extract of the plant can kill the test isolates at concentration of 12.5-50mg/ ml.
Discussion
The phytochemical characteristics in the plant stem bark showed that alkaloid, tannins, saponin, flavonoid, Anthraquinone, terpenoid, phenol and glycoside are present in almost all solvent extracts. Phytochemicals are chemical compounds that are found naturally in plants. They are responsible for the medicinal and organoleptic properties of the plants [21]. Some phytochemicals are responsible for the coloration of the plants and hence determines the medicinal applications of the plant. Phytochemicals are nonnutritive plant chemicals that have protective or disease preventive properties [21]. The phytochemical characteristics possessed by V. doniana may be attributed to their antimicrobial properties. This finding of this study agrees with that conducted by Emmanuel et al. [22] on Phytochemical and Antimicrobial Screening of the Stem- Bark extracts of V. doniana, the results revealed that the preliminary phytochemical screening of the stem back extract contain alkaloids, saponins, tannins, glycosides and flavonoids. The antibacterial activity of aqueous and methanolic stem bark extract of V. doniana against S. aureus and P. aeruginosa recovered from various type of wound showed that the extracts possessed antibacterial activity against the test isolates with methanolic extract showing higher activity compared to aqueous. This is due to better solubility of the phytochemical in organic solvent (methanol) than water. The zone of inhibition shown by the control (50mg/ml Ciprofloxacin) is found to be 15-22 and 17-19mm for S. aureus and P. aeruginosa respectively. The result of this finding demonstrated that the plant extracts are effective against the test isolates. However, the plant extract is more effective on S. aureus when compared to P. aeruginosa. This is due to resistivity of the latter. The result of antibacterial activity of the plant in this study is in line with the study conducted by Kilani [13] to assess antibacterial activity of whole stem back methanolic extract of V. doniana against some members of Enterobacteriaceae including S. typhi, Shigella and E. coli.
The result of his study shows that the stem bark extracts were able to inhibit the growth pattern of the tested microorganisms. According to him, the results suggest that V. doniana may be valuable in the management of dysentery and gastroenteritis infections. The antimicrobial activity of Alkaloids and Flavonoids of Vitex doniana extracts was studied by Okoye [23] in which the result of the study shows that the aqueous extracts of Alkaloids and Flavonoid of the plant inhibited the growth of the following bacteria successfully, P. aeruginosa, B. subtilis, S. aureus, E. coli and S. typhi and this is inconformity with the present study. The minimum inhibitory concentration (MIC) of the stem bark extracts against the test isolates ranges 12.5mg/50 to mg/ml. Low MIC of the extract is due to presence of active biochemical constituents. The MBC of the extracts lies between 12.5mg/ml to 50mg/ml. However, methanolic stem bark extract is found to have lower value of MIC and MBC compared to aqueous extract due to higher antibacterial activity.
Conclusion
The phytochemical screening of V. doniana stem bark extracts indicated the presence of alkaloid, tannins, saponin, flavonoid, Anthraquinone, terpenoid, phenol and glycoside. It is also found that the stem bark extract of V. doniana possessed antibacterial activity against S. aureus and P. aeruginosa recovered from various types of wound. The antibacterial activity of V. doniana stem bark extracts are due to presence of certain bioactive substances called phytochemicals. It is concluded that the stem bark extract of V. doniana can be used as a therapy for wound infection.
References
- Ayton M (1985) Wound care: Wounds that won’t heal. Nurs Times 81(46): 16-19.
- Leaper DJ, Harding KG (1998) Wounds: Biology and management. Oxford University Press, Oxford, UK.
- Rubin RH (2006) Surgical wound infection: epidemiology, pathogenesis, diagnosis and management. BMC Infect Dis 6: 171.
- Tayfour MA, Al Ghamdi SM, Al Ghamd AS (2005) Surgical wound infections in King Fahad Hospital at Al-Baha. Saudi Med J 26(8): 1305- 1307.
- Brook I (1996) Aerobic and anaerobic microbiology of necrotizing fasciitis in children. Pediatr Dermatol 13(4): 281-284.
- Rao R, Sumathi, S, Anuradha K (2013) Bacteriology of postoperative wound infections. Int J pharm biomed res 4(2): 72-76.
- Verma VC, Chitra (2012) Antibiotic sensitivity treatment for gram negative bacteria isolated from pus sample. International Journal of Pharmacy and Biological Sciences 2(3): 359-363.
- Andhoga J, Macharia AG, Maikuma IR, Wanyonyi ZS, Ayumba B, et al. (2002) Aerobic pathogenic bacteria in post-operative wounds at Moi teaching and referral hospital. East Afr Med J 79(12): 640-644.
- Chang HW (1995) Antibacterial effect of spices and vegetables. Food Industries 27: 53-61.
- Del Campo J, Amiot MJ, Nguyen The C (2000) Antimicrobial effect of rosemary extract. J Food Prot 63(10): 1359-1368.
- Dalziel JM, Hutchison J (1955) Useful plants of west tropical Africa. Crown Agent, London, UK.
- Sofowora A (1993) Medicinal plants and traditional medicine in Africa. Spectrum books ltd. (2nd edn). pp. 26-100
- Kilani AM (2006) Antibacterial assessment of whole stem bark of Vitex doniana against some Enterobacteriaceae. African Journal of Biotechnology 5(10): 958-959.
- Adejumo AA, Alaye SA, Ajagbe RO, Abi EA, Adedokun FT (2013) Nutritional and anti-nutritional composition of black-Plum (Vitex Doniana). Journal of Natural Sciences Research 3(12): 144-148.
- Nair R, Chanda SV (2007) Antibacterial activities of some medicinal plants of Western Region of India. Turk J Biol 31: 231-236.
- Cheesbrough M (2000) District laboratory practice in tropical countries. Cambridge United Press, UK, 27: 105.
- Holt JG, Krieg NR, Sneath PA, Stanley JT, Williams ST (1994) Bergey’s manual of systematic bacteriology, (9th edn). Williams & Wilkins Co. Baltimore, Maryland, USA, p. 786.
- Sherman N (2005) Microbiology: A laboratory manual. (6th edn). ISBN 81(3): 265-267.
- Ahmed I, Beg AZ (2001) Antimicrobial and phytochemical studies on 45 Indian Medicinal plants against multi-drug resistance human pathogens. J Ethnopharmacol 74(2): 113-123.
- Anibijuwon II, Udeze OA (2009) Antimicrobial activity of carica papaya (paw-paw leaf) on some pathogenic organisms of clinical origin from south-western, Nigeria. Ethno botanical Leaflets 13: 850-886.
- Doughari JH (2012) Phytochemicals: Extraction methods, basic structures and mode of action as potential chemotherapeutic agents. pp. 1-34.
- Emmanuel IO, Agbafor JI, Omogo S (2015) Phytochemical and antimicrobial screening of the stem-bark extracts of vitex doniana. American-Eurasian Journal of Scientific Research 10(4): 248-250
- Okoye EI (2015) The effect of alkaloids and flavonoid extracts of Vitex doniana seed on some microorganisms. International Journal of Cancer, Clinical Inventions and Experimental Oncology 1(1): 19-23.
© 2018 Ali M. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






