- Submissions

Full Text
Innovation in Tissue Engineering & Regenerative Medicine
A Comprehensive Review on EGFR Targeted Therapies for Non-Small Cell Lung Cancer
Parvatia Dipti1 and Tawil, Bill2*
1Department of Biotechnology, California State University Channel Islands (CSUCI), USA
2Department of Bioengineering, University of California, USA
*Corresponding author:Bill Tawil, Department of Bioengineering, UCLA School of Engineering, 420 Westwood Plaza, Room 5121, Engineering V. P.O. Box: 951600, Los Angeles, CA USA, University of Oklahoma Health Sciences Center, Oklahoma City, OK 73104, USA
Submission: June 06, 2025;Published: August 28, 2025

Volume2 Issue2August 28, 2025
Abstract
Non-Small Cell Lung Cancer (NSCLC) is the largest type of lung cancer, representing around 85% of lung cancer cases worldwide. NSCLC, with a five-year survival rate of only 25%, remains one of the leading causes of cancer-related mortality despite the advances in its early detection. The Epidermal Growth Factor Receptor (EGFR) signaling pathway is one of the central molecular pathways involved in NSCLC pathogenesis. Mutations in the EGFR gene lead to constitutive activation of EGFR, which promotes tumor growth and resists apoptosis, primarily in exon 19 and exon 21. This finding was the basis for a variety of EGFR-directed therapies, and has since transformed the landscape for treatment of NSCLC patients. Tyrosine Kinase Inhibitors (TKIs) specific to EGFR (such as gefitinib, erlotinib, afatinib, and osimertinib) have dramatically enhanced progression-free survival. However, the development of acquired resistance to first- and 2nd -generation EGFR-TKIs frequently occurs through secondary mutations, e.g., T790M. Such mutations have prompted the development of third-generation TKIs, including osimertinib, which provide patients with a more favorable outcome in the face of this resistance. To conclude, this review focuses on the molecular mechanism of EGFR role in NSCLC, history of EGFR-targeted therapies and treatment progress to overcome clinical resistance aimed at improving overall survival in NSCLC patients. Additionally, it discusses the market growth of EGFR inhibitors, key pharmaceutical players, and the importance of precision medicine in shaping the future of lung cancer treatment.
Keywords:Non-Small Cell Lung Cancer (NSCLC); Epidermal Growth Factor Receptor (EGFR); Tyrosine Kinase Inhibitors (TKIs); EGFR mutations; Targeted therapy; Drug resistance; Precision medicine; Lung cancer treatment
Abbreviations:BAT: NSCLC: Non-Small Cell Lung Cancer; EGFR: Epidermal Growth Factor Receptor; TKIs: Tyrosine Kinase Inhibitors; OS: Overall Survival; PFS: Progression-Free Survival; EGFR-TKIs: Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors; T790M: Threonine-to-Methionine Mutation at Codon 790; FDA: Food and Drug Administration; CNS: Central Nervous System; PD: Disease Progression; TKI-R: Tyrosine Kinase Inhibitor Resistance; ALKi: Anaplastic Lymphoma Kinase Inhibitor
Introduction
Lung cancer is still one of the most lethal malignancies internationally with Non-Small Cell Lung Cancer (NSCLC) consisting of ~85% of cases [1]. Each year, more than 170,000 new lung cancer diagnoses occur in the United States, and the malignancy still ranks first in causing cancer-related death in men and women [1-3]. Despite advances in early detection and treatment, lung cancer’s prognosis remains poor, with a five-year survival rate of just 25% for NSCLC [4]. The need for more effective treatments has driven research into the molecular mechanisms driving the disease, leading to the identification of several key oncogenic pathways [4].
One such pathway involves the Epidermal Growth Factor Receptor (EGFR), a transmembrane receptor tyrosine kinase that plays a central role in regulating key cellular functions such as proliferation, survival, migration, and angiogenesis (the formation of new blood vessels) [1]. In normal cells, EGFR activation is tightly controlled by the binding of specific ligands, which leads to receptor dimerization, autophosphorylation, and the initiation of downstream signaling pathways that regulate cell growth and division [5]. However, in many cancers, including NSCLC, mutations in the EGFR gene can result in constitutive activation of the receptor, even in the absence of ligand binding [6,7]. This uncontrolled activation drives tumor growth and progression by promoting continuous cell division, resistance to apoptosis (programmed cell death), and metastasis [8].
In NSCLC, the most common EGFR mutations are the exon 19 deletion (Del19) and the exon 21 L858R point mutation [9]. These mutations are referred to as “sensitizing mutations” because they make tumor cells highly responsive to targeted therapies that inhibit EGFR signaling [1]. These discoveries have transformed the treatment landscape for patients with NSCLC, particularly those whose tumors harbor these mutations, allowing for more personalized and effective therapeutic strategies [10]. Traditional chemotherapy, which targets rapidly dividing cells indiscriminately, has long been the standard treatment for advanced NSCLC [11]. However, chemotherapy is associated with significant side effects, and its efficacy is often limited due to the development of resistance and tumor recurrence [11]. As researchers delved deeper into the molecular underpinnings of NSCLC, it became evident that targeting specific oncogenic drivers, such as EGFR, could offer a more precise and effective approach to treatment, with fewer side effects compared to chemotherapy [12]. EGFR-targeted therapies represent a breakthrough in the management of NSCLC [12]. These therapies are designed to disrupt EGFR signaling, thereby inhibiting tumor growth and progression [12]. The development of drugs that specifically target EGFR has ushered in a new era of precision medicine in oncology, offering patients with EGFR-mutant NSCLC more tailored treatment options with significantly improved outcomes [13].
Market Size, Products, and Companies
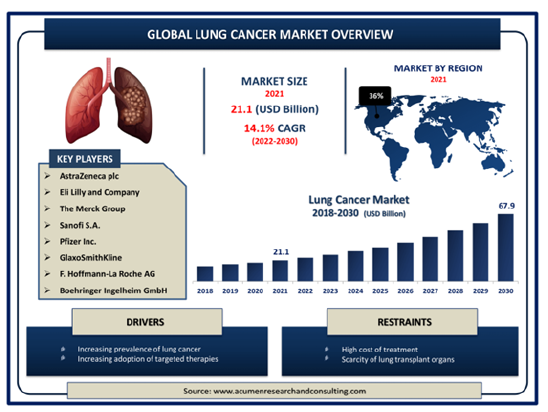
The market for EGFR-targeted therapies in the treatment of Non-Small Cell Lung Cancer (NSCLC) has grown exponentially over the past decade, driven by the increasing incidence of lung cancer, the rise of personalized medicine, and advances in targeted therapies [14]. Globally, lung cancer remains the leading cause of cancer-related deaths, accounting for approximately 2.2 million new cases and 1.8 million deaths annually, with NSCLC representing over 80% of all cases [1,15]. The global market for EGFR-targeted therapies is rapidly expanding, driven by the rising incidence of Non-Small Cell Lung Cancer (NSCLC) and the increasing detection of EGFR mutations in patients [16,17]. In 2021, the market for EGFR inhibitors was valued at USD 21.1 billion and is expected to grow at a Compound Annual Growth Rate (CAGR) of 8-10%, reaching over USD 67.9 billion by 2030 [18,19]. This growth is supported by the shift toward precision medicine, where targeted therapies such as EGFR inhibitors are preferred due to their ability to improve patient outcomes significantly over traditional chemotherapy [18].
Among the leading companies in this space, AstraZeneca plays a crucial role with its range of EGFR-TKIs and one of their most notable products is gefitinib (Iressa), a first-generation EGFR-TKI that targets common EGFR mutations such as Exon 19 deletions and L858R mutations [20-22]. Gefitinib was one of the first therapies to target EGFR mutations and remains widely used, though resistance to the drug often develops over time [9]. AstraZeneca also developed osimertinib (Tagrisso), a third-generation EGFR-TKI that addresses a critical need in patients with the T790M mutation-a resistance mutation that emerges after treatment with first- or secondgeneration EGFR inhibitors [23]. Osimertinib works by irreversibly binding to EGFR mutations while sparing the wild-type EGFR, which reduces off-target effects and improves patient tolerance [24,25]. Osimertinib has become a frontline therapy for advanced NSCLC with EGFR mutations, significantly improving progressionfree survival in patients compared to earlier-generation TKIs [23].
Genentech (Roche) is another major player, particularly with erlotinib (Tarceva), a first-generation EGFR-TKI that remains widely used in EGFR-mutated NSCLC [26]. Like gefitinib, erlotinib blocks EGFR phosphorylation by binding to the ATP-binding site, thereby inhibiting downstream signaling pathways that drive tumor growth [27]. Erlotinib is often evaluated in combination with immunotherapy agents to further enhance treatment efficacy in certain patient populations [24,28]. In the realm of secondgeneration EGFR-TKIs, Boehringer Ingelheim developed afatinib (Gilotrif), which irreversibly inhibits the entire ErbB family of receptors, including EGFR, HER2, and HER4 [29]. Afatinib’s broader action makes it effective in patients with mutations that have become resistant to first-generation therapies like gefitinib and erlotinib [29]. The irreversible binding increases the drug’s potency against aggressive cancers with multiple ErbB mutations [29,30]. The market for third-generation EGFR-TKIs is particularly noteworthy for its focus on overcoming resistance mutations [23]. AstraZeneca’s osimertinib (Tagrisso) is the dominant thirdgeneration drug in the market [23]. Osimertinib targets not only the T790M resistance mutation but also common sensitizing mutations like Exon 19 deletions and L858R [31]. This makes osimertinib particularly valuable as both a first-line and second-line treatment for EGFR-mutated NSCLC [24]. The drug has revolutionized treatment outcomes for NSCLC patients, extending survival rates and delaying disease progression more effectively than earlier therapies [24].
Beyond tyrosine kinase inhibitors, monoclonal antibodies targeting EGFR also contribute to the therapeutic landscape [1]. Monoclonal antibodies work by competing with natural ligands to bind to the outer part of EGFR and this prevents the activation of the EGFR-dependent pathway [1]. In lung cancer trials, these antibodies, like Cetuximab, were tested alone or with chemotherapy [1]. Bristol-Myers Squibb (BMS) and Eli Lilly co-developed cetuximab (Erbitux), an antibody that binds to the extracellular domain of EGFR, blocking its interaction with ligands and thereby preventing downstream signaling [11]. Although cetuximab is more commonly used in colorectal cancer, it also plays a role in NSCLC treatment, particularly in combination with chemotherapy for patients who express wild-type EGFR [11,32].
The emergence of generic manufacturers has further expanded access to EGFR-targeted therapies [33,34]. Companies like Teva Pharmaceuticals, Cipla, Dr. Reddy’s Laboratories, and Mylan (Viatris) produce affordable versions of gefitinib and erlotinib, ensuring that patients worldwide, especially in developing countries, have access to these life-saving drugs [33]. Generic competition has played a crucial role in reducing the cost of first generation EGFR-TKIs, making them more accessible to a broader patient population [16,33].
EGFR-Targeted Therapies in Non-Small Cell Lung Cancer
Epidermal Growth Factor Receptor (EGFR)-targeted therapies have transformed the treatment landscape of Non-Small Cell Lung Cancer (NSCLC), especially in patients harboring specific mutations in the EGFR gene [8]. EGFR is a transmembrane protein involved in regulating cellular proliferation, survival, and differentiation [4,12]. In many cancers, including NSCLC, mutations in the EGFR gene lead to abnormal activation of the receptor, which drives tumor growth [13]. By targeting this receptor, EGFR inhibitors can slow or halt cancer progression [13]. Over time, multiple generations of EGFRtargeted therapies have been developed to overcome both primary tumorigenic activity and resistance mechanisms that cancer cells evolve in response to therapy [35].
The role of EGFR in NSCLC pathogenesis
Before delving into therapies, it’s essential to understand the biological basis for targeting EGFR in NSCLC [4,16]. EGFR belongs to the ErbB family of receptors, which are part of a larger family of Receptor Tyrosine Kinases (RTKs) [5,35]. RTKs play a crucial role in cell signaling pathways that regulate growth, survival, and differentiation [5,35]. Under normal circumstances, EGFR is activated by binding to its ligands, such as Epidermal Growth Factor (EGF) and transforming growth factor-alpha (TGF-α) [12]. Upon ligand binding, EGFR dimerizes with another EGFR molecule or with another member of the ErbB family (HER2, HER3, or HER4), leading to the phosphorylation of tyrosine residues within the intracellular domain [36,37]. This phosphorylation activates several downstream signaling pathways, most notably the RASRAF- MEK-ERK and PI3K-AKT pathways, which are involved in cell proliferation and survival [13,37]. In cancer, activating mutations in the EGFR gene, commonly found in exons 18 to 21, result in constitutive activation of the receptor, even in the absence of ligands [38,39]. These mutations include L858R (a point mutation in exon 21) and deletions in exon 19, which are the most common in NSCLC [40]. The result is uncontrolled cellular proliferation and tumorigenesis [40]. This insight into the molecular underpinnings of EGFR’s role in NSCLC led to the development of therapies designed to inhibit its function [10].
Tyrosine Kinase Inhibitors (TKIs)
The introduction of EGFR Tyrosine Kinase Inhibitors (TKIs) marked a paradigm shift in the treatment of EGFR-mutated NSCLC [13]. TKIs work by binding to the intracellular kinase domain of EGFR, blocking its ability to phosphorylate tyrosine residues and activate downstream signaling pathways that promote cancer cell growth and survival [5,35]. Over the years, the development of EGFR TKIs has progressed through multiple generations, each designed to improve efficacy and overcome resistance mechanisms that cancer cells acquire during treatment [41].
First-Generation TKIs: First-generation TKIs, such as gefitinib (Iressa) and erlotinib (Tarceva), were the first to target EGFR mutations in NSCLC [42]. These drugs are small molecules that reversibly bind to the ATP-binding site of the EGFR tyrosine kinase domain [36]. By competing with ATP for binding, gefitinib and erlotinib inhibit EGFR autophosphorylation and the subsequent activation of downstream signaling pathways, including the RASRAF- MEK-ERK and PI3K-AKT-mTOR pathways [26,27]. These pathways are critical for the growth, survival, and proliferation of cancer cells [9]. The initial success of gefitinib and erlotinib in clinical trials led to their approval for the treatment of patients with advanced NSCLC harboring specific EGFR mutations [20- 22]. Clinical studies demonstrated that patients with activating EGFR mutations, such as deletions in exon 19 and the L858R point mutation in exon 21, responded exceptionally well to these TKIs, achieving high rates of response and longer Progression- Free Survival (PFS) compared to standard chemotherapy [12,8]. However, despite the initial efficacy, most patients experienced disease progression after a median of 9-12 months of therapy [8]. This acquired resistance is primarily due to the emergence of a secondary mutation in EGFR, known as T790M [13]. This mutation alters the ATP-binding pocket of EGFR, increasing its affinity for ATP and diminishing the binding of first-generation TKIs [5,35].
Second-Generation TKIs: To overcome resistance to firstgeneration TKIs, particularly the T790M mutation, secondgeneration EGFR inhibitors, such as afatinib (Gilotrif) and dacomitinib, were developed [29,43]. These drugs are designed to bind irreversibly to the ATP-binding site of the EGFR kinase domain, forming a covalent bond that results in prolonged inhibition of the receptor [43,44]. In addition to targeting EGFR, these secondgeneration inhibitors also inhibit other members of the ErbB family, such as HER2 and HER4, which are often implicated in the activation of alternative growth pathways in cancer cells [29,43]. Afatinib has shown efficacy in patients with common EGFR mutations (Del19 and L858R) as well as in patients with rarer EGFR mutations [29]. It provides a more durable response due to its irreversible binding mechanism, which prevents reactivation of the receptor [29]. However, the broader inhibitory activity of second-generation TKIs also leads to a higher incidence of side effects, particularly skin rashes and diarrhea, which can be more severe compared to first-generation TKIs [43]. One of the limitations of secondgeneration TKIs is that while they can overcome certain resistance mechanisms, they are not as effective against the T790M mutation, which remains the most common cause of resistance to EGFRtargeted therapies [35]. This limitation led to the development of third-generation TKIs that specifically target T790M [45].
Third-Generation TKIs: Third-generation TKIs, such as osimertinib (Tagrisso), represent a significant advancement in the treatment of EGFR-mutated NSCLC [24,35]. These inhibitors are designed to selectively target both activating EGFR mutations and the T790M resistance mutation while sparing wild-type EGFR, thereby reducing off-target toxicities [23]. Osimertinib binds covalently to the ATP-binding pocket of the mutated EGFR kinase, providing potent and selective inhibition [43,44]. Clinical trials, such as the AURA3 trial, demonstrated the efficacy of osimertinib in patients with T790M-mediated resistance to first- or secondgeneration TKIs [46]. The trial showed that osimertinib significantly improved progression-free survival compared to platinum-based chemotherapy in patients who had developed resistance [46]. Furthermore, the FLAURA trial established osimertinib as a firstline treatment for patients with EGFR-mutated NSCLC, showing superior overall survival and progression-free survival compared to first-generation TKIs like gefitinib and erlotinib [46].
Osimertinib has since become the standard of care for EGFRmutant NSCLC patients, both in the first-line setting and for those who develop T790M-mediated resistance [13,43]. Despite its success, resistance to osimertinib eventually emerges, often through mechanisms that are independent of EGFR, such as MET amplification, HER2 amplification, or transformation to Small-Cell Lung Cancer (SCLC) [45]. As a result, ongoing research is focused on developing fourth-generation TKIs and combination therapies to address these resistance mechanisms [47].
Monoclonal antibodies targeting EGFR
In addition to TKIs, Monoclonal Antibodies (mAbs) targeting the extracellular domain of EGFR have been developed as another therapeutic approach in NSCLC [24]. Unlike TKIs, which inhibit the intracellular kinase activity of EGFR, monoclonal antibodies prevent ligand binding and receptor dimerization, thereby inhibiting EGFR activation [11]. Cetuximab (Erbitux) is the most well-known EGFRtargeting monoclonal antibody [24]. It has shown limited efficacy in NSCLC when used as a monotherapy but has demonstrated benefit in combination with chemotherapy [11]. Cetuximab binds to the extracellular domain of EGFR, preventing the receptor from dimerizing and becoming activated [48]. It also induces receptor internalization and degradation, thereby reducing the number of EGFR molecules available on the cell surface [48,49]. cetuximab has been studied in combination with chemotherapy and radiotherapy for the treatment of advanced NSCLC, although its use is not as widespread as TKIs [49].
Ongoing Studies and Clinical Trials
The development of fourth-generation EGFR-TKIs (epidermal growth factor receptor tyrosine kinase inhibitors) marks a promising advance in targeted cancer therapy, particularly for patients With Non-Small Cell Lung Cancer (NSCLC) [47]. As resistance to existing EGFR-TKIs, such as first-generation agents (gefitinib, erlotinib) and third-generation agents (osimertinib), becomes increasingly prevalent, the focus has shifted to developing novel therapeutic strategies [13,50,51]. Companies like Blueprint Medicines are at the forefront of this evolution, with their experimental drug, BLU-945, demonstrating significant potential [52]. BLU-945 is specifically designed to target mutations associated with acquired resistance, such as C797S [52,53]. This particular mutation arises from the initial treatment with osimertinib, which, while effective, does not provide a durable response in all patients [52,53]. The emergence of resistant mutations underscores the need for innovative therapeutic options that can bypass or overcome these resistance mechanisms [50]. Clinical trials investigating BLU-945 and similar compounds are currently underway, focusing on their safety, efficacy, and overall impact on disease control [54]. Preliminary studies have shown promising results, suggesting that BLU-945 could offer enhanced activity against resistant tumors compared to third-generation TKIs [54]. The ongoing clinical trials aim to recruit patients who have experienced disease progression after treatment with prior EGFR-TKIs, thereby directly addressing a significant unmet need in the NSCLC patient population [54].
The potential advantages of fourth-generation EGFR-TKIs extend beyond simply targeting resistance mutations [47,54]. These drugs are designed to minimize off-target effects and improve patient tolerability, allowing for prolonged treatment durations without compromising quality of life [54]. By offering new mechanisms of action, fourth-generation agents may also work synergistically with other treatment modalities, such as immune checkpoint inhibitors, paving the way for combination therapies that could improve patient outcomes [55-58].
Furthermore, the landscape of targeted therapy is evolving with the advent of comprehensive genomic profiling in clinical practice [12]. This approach enables clinicians to identify specific mutations driving a patient’s cancer, facilitating personalized treatment strategies that align with the unique molecular characteristics of their tumors [12]. As ongoing studies continue to yield data on the efficacy of BLU-945 and other fourth-generation EGFR-TKIs, it is anticipated that these agents will play a critical role in the management of NSCLC, particularly in patients with resistance to earlier treatments [52,53]; (Figures 1-6).
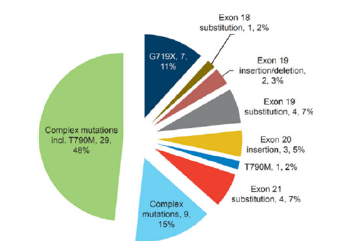
Fgure 1:Distribution of the 60 rare EGFR mutations [59]. This image shows the distribution of 60 rare EGFR mutations, with each segment representing a mutation and its frequency in the cohort.
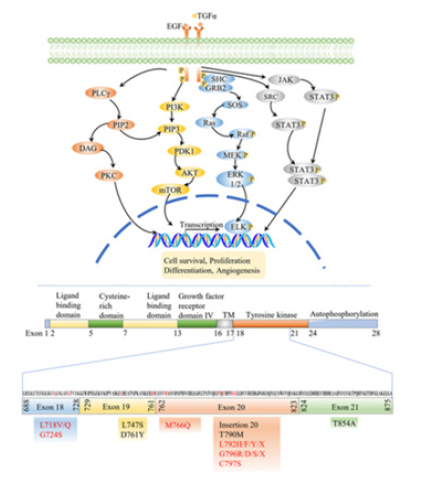
Fgure 2:EGFR signaling pathways and major mutations of EGFR to TKIs resistance [53]. This image shows (A) EGFR activates four key survival pathways. (B) Black font shows mutations resistant to firstand second-generation TKIs, while red font indicates resistance to third-generation TKI osimertinib.
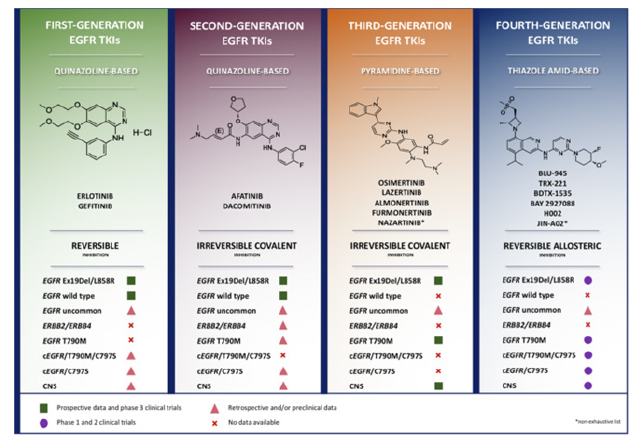
Fgure 3:Comparative overview of EGFR tyrosine-kinase inhibitors [53]. This image shows the overview of the four classes/generations of EGFR inhibitors, highlighting their distinct molecular structures, mechanisms of action, and activities.
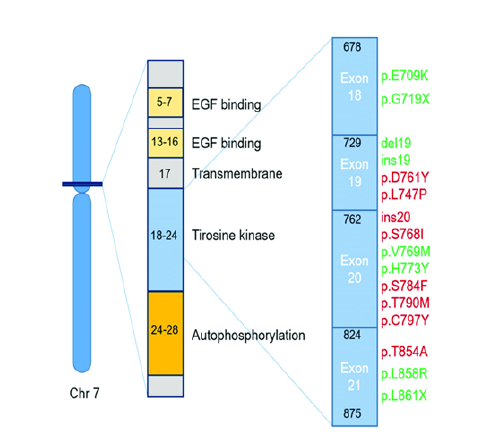
Fgure 4:Impact of Common EGFR Mutations on TKI Response in Non-Small Cell Lung Cancer: Sensitivity and Resistance [60]. Effect of the most common EGFR mutations in NSCLC (Non-Small-Cell Lung Cancer) on the EGFR TKI (Tyrosine Kinase Inhibitor) response. Schematic representation of the location of the most frequent EGFR mutations in NSCLC and their relationship to resistance (red) and sensitivity (green) in the treatment with EGFR TKIs.
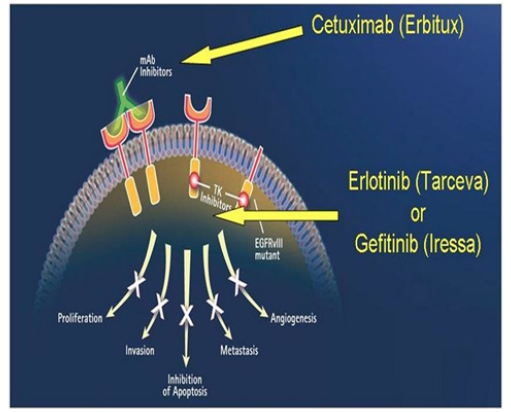
Fgure 5:Mechanisms of Action of EGFR Inhibitors: Monoclonal Antibodies and TKIs [61]. EGFR inhibitors and their mode of action Cetuximab, mAb, binds to the extracellular domain of the EGFR receptors, while Erlotinib and Gefitinib, TKIs, bind to the tyrosine kinase domain.
Fgure 6:Lung Cancer Market Size [62]. Lung Cancer Market Size - Global Industry, Share, Analysis, Trends and Forecast 2022 – 2030.
Conclusion and Future Direction
The advent of EGFR-targeted therapies has transformed the treatment landscape for Non-Small Cell Lung Cancer (NSCLC), particularly in patients with EGFR mutations. These therapies, including Tyrosine Kinase Inhibitors (TKIs) such as gefitinib, erlotinib, and osimertinib, have significantly improved outcomes for patients by offering a more personalized approach compared to traditional chemotherapy. The development of these drugs has led to longer progression-free survival and better overall survival rates, especially in patients with sensitizing mutations like the exon 19 deletion and L858R point mutation. However, despite the initial success of these targeted therapies, the emergence of resistance mutations, most notably T790M, continues to challenge long-term treatment efficacy. Third-generation TKIs, such as osimertinib, have shown great promise in overcoming resistance mutations, particularly T790M, and are now considered the standard of care for advanced EGFR-mutant NSCLC. The success of these therapies has solidified the role of EGFR inhibitors in precision medicine, providing hope for further advancements in the treatment of NSCLC. However, as cancer cells inevitably develop new resistance mechanisms, the need for novel therapeutic strategies remains critical.
While EGFR-targeted therapies have undoubtedly revolutionized the management of NSCLC, several challenges and opportunities for future research remain. The ongoing emergence of resistance mutations necessitates the development of new treatment options that can either prevent or effectively manage resistance. Future research should focus on identifying additional resistance mechanisms beyond T790M, including mutations in other pathways or gene amplifications that allow cancer cells to bypass EGFR inhibition. One promising direction is the combination of EGFR-targeted therapies with other treatment modalities, such as immunotherapy or inhibitors of alternative pathways like MET, HER2, or RET. Studies combining TKIs with immune checkpoint inhibitors or monoclonal antibodies are ongoing and could further enhance treatment efficacy and delay resistance. Additionally, identifying biomarkers that predict response to combination therapies will be crucial in tailoring treatments more effectively. Another critical area of research is the development of nextgeneration TKIs with broader specificity and improved safety profiles. These inhibitors should not only target known resistance mutations but also minimize off-target effects, reducing toxicity and improving patient quality of life.
Finally, as personalized medicine continues to evolve, the integration of genomic and proteomic profiling into routine clinical practice will allow for even more precise targeting of oncogenic drivers in NSCLC. Advances in liquid biopsy technology may provide non-invasive methods for monitoring tumor evolution in real time, allowing for early detection of resistance mutations and timely adjustments in treatment strategy. In conclusion, while EGFR-targeted therapies have significantly improved outcomes for NSCLC patients, ongoing research is vital to overcoming the limitations posed by drug resistance and expanding the scope of precision oncology in lung cancer treatment.
Acknowledgement
Dipti Parvatia expresses appreciation to Professor Bill Tawil for overseeing the framework of this review, and for the insightful lectures which contributed to the development of this paper.
References
- Pallis AG, Serfass L, Dziadziusko R, Meerbeeck JP, Fennell D, et al. (2009) Targeted therapies in the treatment of advanced/metastatic NSCLC. Eur J Cancer 45(14): 2473-2487.
- Thai AA, Solomon BJ, Sequist LV, Gainor JF, Heist RS (2021) Lung cancer. Lancet 398(10299): 535-554.
- Harrison PT, Vyse S, Huang PH (2020) Rare Epidermal Growth Factor Receptor (EGFR) mutations in non-small cell lung cancer. Semin Cancer Biol 61: 167-179 .
- Levantini E, Maroni G, Re M, Tenen DG (2022) EGFR signaling pathway as therapeutic target in human cancers. Semin Cancer Biol 85: 253-275.
- Gelatti ACZ, Drilon A, Santini FC (2019) Optimizing the sequencing of Tyrosine Kinase Inhibitors (TKIs) in Epidermal Growth Factor Receptor (EGFR) mutation-positive Non-Small Cell Lung Cancer (NSCLC). Lung Cancer 137: 113-122.
- Raman V, Yang CFJ, Deng JZ, D'Amico TA (2018) Surgical treatment for early stage non-small cell lung cancer. J Thorac Dis 10(7): S898-S904.
- Midha A, Dearden S, McCormack R (2015) EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: A systematic review and global map by ethnicity (mutMapII). Am J Cancer Res 5(9): 2892-2911.
- Lei Y, Wang K, Liu Y, Wang X, Xiang X, et al. (2022) Various subtypes of EGFR mutations in patients with NSCLC Define genetic, immunologic diversity and possess different prognostic biomarkers. Front Immunol 13: 811601.
- Chunsheng Wang, Kewei Zhao, Shanliang Hu, Wei Dong, Yan Gong, et al. (2022) Clinical outcomes of gefitinib and erlotinib in patients with NSCLC harboring uncommon EGFR mutations: A pooled analysis of 438 patients. Lung Cancer 172: 86-93.
- Uprety D, Mandrekar SJ, Wigle D, Roden AC, Adjei AA (2020) Neoadjuvant immunotherapy for NSCLC: Current concepts and future approaches. J Thorac Oncol 15: 1281-1297.
- Hao Lin, Jingwei Jiang, Xiaohua Liang, XinLi Zhou, Ruofan Huang (2010) Chemotherapy with cetuximab or chemotherapy alone for untreated advanced non-small-cell lung cancer: A systematic review and meta-analysis, Lung Cancer 70(1): 57-62.
- Chen N, Fang W, Zhan J, Hong S, Tang Y, et al. (2015) Upregulation of PD-L1 by EGFR activation mediates the immune escape in EGFR-driven NSCLC: implication for optional immune targeted therapy for NSCLC patients with EGFR mutation. J Thorac Oncol 10(6): 910-923.
- Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, et al. (2011) Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR mutant lung cancer: Distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 17(6): 1616-1622.
- Philip Baum, Rafael Cardoso, Jacopo Lenzi, Damhuis RAM, Verhagen FTM, et al. (2024) An International registry study of early-stage NSCLC treatment variations (LUCAEUROPE) in Europe and the USA highlighting variations. European Journal of Cancer 209: 114233.
- Sharma R (2022) Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol 27(4): 665-675.
- Graham RP, Treece AL, Lindeman NI, Patricia Vasalos, Mu Shan, et al. (2017) Worldwide frequency of commonly detected EGFR mutations. Arch Pathol Lab Med 142(2): 163-167.
- Cho JH, Zhou W, Choi YL, Sun J, Choi H, et al. (2018) Retrospective molecular epidemiology study of PD-L1 expression in patients with EGFR-mutant non-small cell lung cancer. Cancer Res Treat 50(1): 95-102.
- Adchata Konsue, Thomanai Lamtha, Duangkamol Gleeson, Jones D, Britton RG, et al. (2024) Design, preparation and biological evaluation of new Rociletinib-inspired analogs as irreversible EGFR inhibitors to treat non-small-cell-lung cancer. Bioorganic & Medicinal Chemistry 113: 117906.
- Lung cancer market size - Global industry, share, analysis, trends and forecast 2022-2030. pp. 1-250
- Wang Y, Wei J, Feng L, Li O, Huang L, et al. (2023) Aberrant m5C hypermethylation mediates intrinsic resistance to gefitinib through NSUN2/YBX1/QSOX1 axis in EGFR-mutant non-small-cell lung cancer. Mol Cancer 22(1): 81.
- Sandra Gottschling, Esther Herpel, Eberhardt WEE, Heigener DF, Fischer JR, et al. (2012) The gefitinib long-term responder (LTR)-A cancer stem-like cell story? Insights from molecular analyses of German long-term responders treated in the IRESSA expanded access program (EAP). Lung Cancer 77(1): 183-191.
- Nancy Price, Belani CP (2005) Clinical development of gefitinib in non-small-cell lung cancer and the Iressa® survival evaluation in lung cancer trial. clinical Lung Cancer 6(4): 214-216.
- Wu YL, Tsuboi M, He J, John T, Grohe C, et al. (2020) Osimertinib in Resected EGFR-Mutated non-small-cell lung cancer. N Engl J Med 383(18): 1711-1723.
- Ilaria Marrocco, Suvendu Giri, Arturo Simoni-Nieves, Nitin Gupta, Anna Rudnitsky, et al. (2023) L858R emerges as a potential biomarker predicting response of lung cancer models to anti-EGFR antibodies: Comparison of osimertinib vs. cetuximab. Cell Reports Medicine 4(8): 101142.
- Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, et al. (2018) Osimertinib in untreated EGFR -mutated advanced non–small-cell lung cancer. N Engl J Med 378(2): 113-125.
- Rocha-Lima CM, Raez LE (2009) Erlotinib (tarceva) for the treatment of non-small-cell lung cancer and pancreatic cancer. P T 34(10): 554-564.
- Greenhalgh J, Bagust A, Boland A, Dwan K, Beale S, et al. (2015) Erlotinib and gefitinib for treating non-small cell lung cancer that has progressed following prior chemotherapy (review of NICE technology appraisals 162 and 175): A systematic review and economic evaluation. Health Technol Assess 19(47): 1-134.
- Shi C, Wang Y, Xue J, Zhou X (2022) Immunotherapy for EGFR-mutant advanced non-small cell lung cancer: current status, possible mechanisms and application prospects. Front Immunol 13: 940288.
- Deeks ED, Keating GM (2018) Afatinib in advanced NSCLC: A profile of its use. Drugs Ther Perspect 34(3): 89-98.
- Xia C, Yin S, To KKW, Fu L (2023) CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer 22: 44.
- Wu YL, Tsuboi M, He J, John T, Grohe C, et al. (2020) Osimertinib in resected EGFR -mutated non–small-cell lung cancer. N Engl J Med 383(18): 1711-1723.
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA (2008) Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 83: 584-594.
- Melosky B, Kambartel K, Häntschel M, Bennetts M, Nickens DJ, et al. (2022) Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: A meta-analysis. Mol Diagn Ther 26(1): 7-18.
- Grant C, Hagopian G, Nagasaka M (2023) Neoadjuvant therapy in non-small cell lung cancer. Crit Rev Oncol Hematol 190:104080.
- Uehara Y, Takeyasu Y, Yoshida T, Tateishi A, Torasawa M, Y, et al. (2024) Real-world outcomes of treatment strategy between first-line osimertinib, first/second-generation EGFR-TKIs followed by Osimertinib, and without osimertinib in advanced EGFR-mutant NSCLC. ESMO Real World Data and Digital Oncology 5: 100058.
- Chung FT, Lee KY, Wang CW, Heh C, Chan Y, et al. (2012) Tumor-associated macrophages correlate with response to epidermal growth factor receptor-tyrosine kinase inhibitors in advanced non-small cell lung cancer. Int J Cancer 131(3): E227-E235.
- Krause DS, Etten RAV (2005) Tyrosine kinases as targets for cancer therapy. N Engl J Med 353(2): 172-187.
- Cunha SG, Shepherd FA, Mutations TMSE (2011) EGFR mutations and lung cancer. 6: 49-69.
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, et al. (2010) Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 11: 121-128.
- Wu YL, Cheng Y, Zhou X, Lee HK, Nakagawa K, et al. (2017) Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 18: 1454-1466.
- Yi Dong, Liaqat Khan, Yi Yao (2024) Immunological features of EGFR-mutant non-small cell lung cancer and clinical practice: A narrative review. Journal of the National Cancer Center 4(4): 289-298.
- Sheng J, Fang W, Liu X, Xing S, Zhan J, et al. (2017) Impact of gefitinib in early stage treatment on circulating cytokines and lymphocytes for patients with advanced non-small cell lung cancer. Onco Targets Ther 10: 1101-1110 .
- Popat S, Jung HA, Lee SY, Hochmair MJ, Lee SH, et al. (2021) Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global non-interventional study (UpSwinG). Lung Cancer 162: 9-15.
- Tozuka T, Noro R, Mizutani H, Kurimoto F, Taiki Hakozaki, et al. (2024) Osimertinib plus local treatment for brain metastases versus osimertinib alone in patients with EGFR mutant non-small cell lung cancer. Lung Cancer 191: 107540.
- Greig SL (2016) Osimertinib: First global approval. Drugs 76(2): 263-273.
- Gray JE, Markovets A, Reungwetwattana T, Majem M, Nogami N, et al. (2024) Longitudinal analyses of circulating tumor DNA for the detection of EGFR mutation-positive advanced NSCLC progression during treatment: Data from FLAURA and AURA3. Journal of Thoracic Oncology 19(11): 1525-1538.
- Carla Corvaja, Antonio Passaro, Ilaria Attili, Aliaga PT, Gianluca Spitaleri, et al. (2024) Advancements in fourth-generation EGFR TKIs in EGFR-mutant NSCLC: Bridging biological insights and therapeutic development. Cancer Treatment Reviews 130: 102824.
- Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, et al. (2016) Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34(25): 2980-2987.
- Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, et al. (2049) Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: Results from the phase I KEYNOTE-001 study. J Clin Oncol 37(28): 2518-
- Passarelli A, Aieta M, Sgambato A, Gridelli C (2020) Targeting immunometabolism mediated by CD73 pathway in EGFR-mutated non-small cell lung cancer: A new hope for overcoming immune resistance. Front Immunol 11: 1479.
- Madeddu C, Donisi C, Liscia N, Lai E, Scartozzi M, et al. (2022) EGFR-mutated non-small cell lung cancer and resistance to immunotherapy: Role of the tumor microenvironment. Int J Mol Sci 23(12): 6489.
- Eno MS, Brubaker JD, Campbell JE, Savi CD, Guzi TJ, et al. (2022) Dineen, discovery of BLU-945, a reversible, potent, and wild-type-sparing next-generation EGFR mutant inhibitor for treatment-resistant non-small-cell lung cancer. Journal of Medicinal Chemistry 65(14): 9662-9677.
- Elamin Y, Nagasaka M, Shum E, Bazhenova L, Rotow J, et al. (2023) BLU-945 monotherapy and combination with osimertinib in previously treated patients with advanced EGFR-Mutant (EGFRm) NSCLC in Phase 1/2 SYMPHONY study. The Journal of Liquid Biopsy 1: 100125.
- Zhou H, Fu H, Shao X, Cai W (2023) Binding thermodynamics of fourth-generation EGFR inhibitors revealed by absolute binding free energy calculations. J Chem Inf Model 63(24): 7837-7846.
- Havel JJ, Chowell D, Chan TA (2019) The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 19(3): 133-150.
- Qiao M, Jiang T, Liu X, Mao S, Zhou F, et al. (2021) Immune checkpoint inhibitors in EGFR-mutated NSCLC: dusk or dawn? J Thorac Oncol 16(8): 1267-1288.
- Lee CK, Man J, Lord S, Links M, Gebski V, et al. (2017) Checkpoint inhibitors in metastatic EGFR-mutated non-Small cell lung cancer-A meta-analysis. J Thorac Oncol 12(2): 403-407.
- Lim SM, Fujino T, Kim C, Lee G, Lee YH, et al. (2023) BBT-176, a novel fourth-generation tyrosine kinase inhibitor for osimertinib-resistant EGFR mutations in non-small cell lung cancer. Clin Cancer Res 29(16): 3004-3016.
- David H, Christian S, Martin S, Parvis S, Ingo S, et al. (2015) Afatinib in non-small cell lung cancer harboring uncommon EGFR mutations pretreated with reversible EGFR inhibitors. The Oncologist 20(10): 1167-1174.
- Dong RF, Zhu ML, Liu MM, Xu YT, Yuan LL, et al. (2021) EGFR mutation mediates resistance to EGFR tyrosine kinase inhibitors in NSCLC: From molecular mechanisms to clinical research. Pharmacological Research 167: 105583.
- Daniela F, Juliana M, Paula ML, Filomena A, Raquel C (2021) Future perspectives in detecting EGFR and ALK gene alterations in liquid biopsies of patients with NSCLC. International Journal of Molecular Sciences 22(8): 3815.
- Ankush C (2015) The Role of ErbB3 Inhibitors as Cancer Therapeutics. pp. 1-80.
© 2025 Tawil, Bill. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






