- Submissions

Full Text
Intervention in Obesity & Diabetes
Pivotal Roles of Diacylglycerol O-Acyltransferase 1 (DGAT1) and Carbonic Anhydrase Enzymes in Obesity and Diabetes
Ghulam Abbas* and Quazi M I Haq*
Department of Biological Sciences and Chemistry, University of Nizwa, Oman
*Corresponding author:Ghulam Abbas and Quazi M I Haq, Department of Biological Sciences and Chemistry, University of Nizwa, P.O. Box 33, PC 616, Nizwa, Oman
Submission:April 26, 2023;Published: May 17, 2023

ISSN 2578-0263Volume6 Issue2
Abstract
All around the world, the prevalence of diabetes mellitus is rising at an alarming rate. Obesity has been found to be crucial in the management of diabetes mellitus because it prolongs the period between becoming pre-diabetic and developing diabetes. The development of obesity is brought on by the buildup of lipids in adipocytes, which is facilitated by the Diacylglycerol O-Acyltransferase 1 (DGAT1). Increased DGAT1 levels in adipose tissue have been linked to insulin resistance and adipocyte hypertrophy in obese individuals. Diarylglycerol O-Acyltransferase 1 (DGAT1) is a key player in the control of diabetes and obesity. There are 16 known human carbonic anhydrase isoforms, and CA IX has also been linked to diabetes and obesity. According to research, obese people have higher levels of CA IX expression in their adipose tissue. CA IX inhibition has been demonstrated to diminish adipocyte differentiation and fat storage in vitro. The main functions of two pharmacologically significant enzymes, DGAT1 and carbonic anhydrase, are discussed in this review article. To demonstrate the potential of these enzymes as anti-obesity and anti-diabetic targets, some prospective inhibitors and various adverse effects of anti-obesity drugs are also discussed.
Keywords:Diacylglycerol O-acyltransferase 1; Carbonic anhydrase; CA IX isoform; Obesity; Diabetes
Introduction
Diabetes Mellitus (DM) is a chronic metabolic disorder in which human body cannot utilize glucose properly owing to decreased insulin secretion or absence of insulin responsiveness or both of them. Despite of the induction of new types of drugs and approaches still global diabetes crises has not been addressed. Various studies assessed that 8.8% of the world’s population between the ages of 20-79 are suffering from diabetes [1-3]. There is convincing evidence that controlling obesity helps to manage type-2 diabetes by delaying from pre-diabetic to become diabetic and thus is extremely helpful in treating type-2 diabetes [4,5]. Modest and sustained weight loss has been demonstrated to improve glycemic control and lessen the requirement for glucose-lowering drugs in individuals with type-2 diabetes who are also overweight or obese [6]. More extensive dietary energy restriction with very-low-calorie diets has been shown to significantly lower A1C and fasting glucose in patients with type-2 diabetes and obesity and to induce sustained diabetic remission for at least two years [7-9]. Novel, efficient therapeutic approaches are highly inevitable to address these problems of global concern [10].
Role of Diacylglycerol O-Acyltransferase 1 (DGAT1) in Obesity and Diabetes
Diacylglycerol O-acyltransferase 1 (DGAT1) is an enzyme that plays a key role in triglyceride synthesis. It is a member of the DGAT family of enzymes and is primarily expressed in the small intestine, adipose tissue, and the liver [11,12]. DGAT1 catalyzes the final step in the synthesis of triglycerides, which involves the transfer of an acyl group from acyl-CoA to Diacylglycerol (DAG) to form a Triacylglycerol (TAG) molecule. This reaction is important for the storage of energy in adipose tissue and the production of lipoproteins in the liver [13,14]. DGAT1 has been the focus of research as a potential therapeutic target for the treatment of obesity, type 2 diabetes, and other metabolic disorders. Several DGAT1 inhibitors have been developed and tested in preclinical and clinical studies, with promising results. Additionally, DGAT1 gene variants have been associated with altered lipid metabolism and risk of metabolic diseases in humans [15-17]. DGAT1 is also found in milk and is important for the synthesis of milk fat. Inhibition of DGAT1 in dairy cows has been shown to reduce milk fat content, which could have implications for the dairy industry [18-20].
DGAT1 enzyme plays its vital role in the synthesis of Triacylglycerol (TAG) from Diacylglycerol (DAG) and fatty acyl-CoA. This enzyme is found in many tissues, including adipose tissue, liver, and intestine, and is known to be up regulated in obesity and type 2 diabetes [21-23]. In obesity, increased levels of DGAT1 in adipose tissue have been shown to contribute to adipocyte hypertrophy and insulin resistance. Specifically, DGAT1 promotes the synthesis of TAG, which leads to the accumulation of lipids in adipocytes and the subsequent development of obesity. This lipid accumulation is thought to contribute to the development of insulin resistance, as the excess lipid can interfere with insulin signalling pathways [24- 26]. In addition to its role in obesity, DGAT1 has also been implicated in the development of type 2 diabetes. Studies have shown that DGAT1 expression is upregulated in the liver of obese and diabetic mice and humans, and that this upregulation is associated with increased hepatic lipid accumulation, inflammation, and insulin resistance. Furthermore, genetic deletion of DGAT1 in mice has been shown to improve glucose homeostasis and insulin sensitivity, highlighting the potential therapeutic benefits of targeting DGAT1 for the treatment of type 2 diabetes [27-30].
Identification of DGAT1 inhibitors by Tschapalda and coworker
Tschapalda and colleagues investigated hundreds of lipid storage inhibitors in a study and discovered three structurally diverse and powerful chemical classes that were active in cells of many species, including human cells, as DGAT1 inhibitors with low cytotoxicity, as shown in Figure 1. Compounds 4,5 and 6 the analogues of compounds 1, 2 and 3 exhibited potent DGAT1 inhibitory activity with EC50 values of 0.34nM, 9nM and 250nM, respectively [31].
Figure 1: Potent DGAT 1 inhibitors.

Identification of DGAT1 inhibitors by Huang and coworkers
In another study, Huang and co-workers assessed the prospective therapeutic effects of DGAT1 inhibitors 7 and 8 (Figure 2) on pancreatic β-cells, and further proved their antidiabetic effects in db/db mice. This study confirmed that DGAT1 inhibitors compounds 7 and 8 have significantly reduced the apoptosis of pancreatic islets in db/db mice and considerably decreased fasting blood glucose and triglyceride levels [32]. Overall, DGAT1 plays an important role in the development of obesity and type 2 diabetes, primarily through its role in promoting the synthesis of TAG and the subsequent accumulation of lipids in adipose tissue and liver. Targeting DGAT1 may therefore be a promising therapeutic approach for the treatment of these metabolic disorders.
Figure 2:DGAT1 inhibitors with antidiabetic effects on obese diabetic db/db mice.

Role of Carbonic Anhydrase Enzymes in Obesity and Diabetes
A group of metalloenzymes known as Carbonic Anhydrases (CAs) catalyze the reversible conversion of carbon dioxide (CO2) into bicarbonate (HCO3-) and protons (H+). Numerous physiological systems, including acid-base balance, respiration, and electrolyte transport, depend on this interaction [33,34]. Carbonic anhydrases are found in many tissues, including the lungs, kidneys, liver, and pancreas, and have been identified in various organisms, including bacteria, algae, and animals [35]. There are currently 16 known isoforms of carbonic anhydrases in humans, which are classified into three main groups: cytosolic (CA I, II, III, VII, XIII, and XV), mitochondrial (CA VA and VB), and membrane-bound (CA IV, IX, XII, XIV, and XVII-XVIII) [36]. Carbonic anhydrases have been the subject of extensive research due to their importance in various physiological processes and their potential as therapeutic targets for a range of diseases, including glaucoma, cancer, and epilepsy. Carbonic anhydrase inhibitors are also used clinically for the treatment of conditions such as glaucoma, altitude sickness, and epilepsy [37,38].
There is emerging evidence suggesting a potential role for Carbonic Anhydrases (CAs) in the development of obesity and diabetes. Some studies have shown that the expression levels of certain isoforms of CAs are altered in obese and diabetic individuals compared to healthy controls. One isoform that has been implicated in obesity and diabetes is CA III. Studies have shown that the expression of CA III is increased in the skeletal muscles of obese and diabetic individuals and that this increase may contribute to insulin resistance and impaired glucose metabolism. Furthermore, inhibition of CA III has been shown to improve glucose uptake in skeletal muscle cells and improve glucose tolerance in obese and diabetic animal models [39,40]. Another isoform, CA IX, has also been implicated in obesity and diabetes. Studies have shown that CA IX expression is increased in adipose tissue of obese individuals, and that CA IX inhibition can reduce adipocyte differentiation and fat accumulation in vitro [41].
Identification of CAs inhibitors by Huang and co-workers
Costa and co-workers for the first time investigated essential oils against mitochondrial isoform VA of the carbonic anhydrase enzyme [42]. Prior to this a structure-based virtual screening was conducted to identify new anti-obesity drug. As a result, some active compounds (9-14) of essential oils were identified with anti-obesity potential against three isoforms of carbonic anhydrase enzyme in vitro, as shown in Table 1. In this study, new scaffold was identified that will be useful in optimization studies to develop novel anti-obesity drugs as shown in Figure 3. The role of CAs in obesity and diabetes is still being elucidated, there is growing evidence to suggest that these enzymes may be involved in the pathogenesis of these diseases and may be potential targets for therapeutic intervention. Overall, carbonic anhydrases are important enzymes with a wide range of physiological roles, and their study has significant implications for both basic science and clinical applications.
Table 1: Active components of essential oils as inhibitors of carbonic anhydrase as per potential anti-obesity drugs.
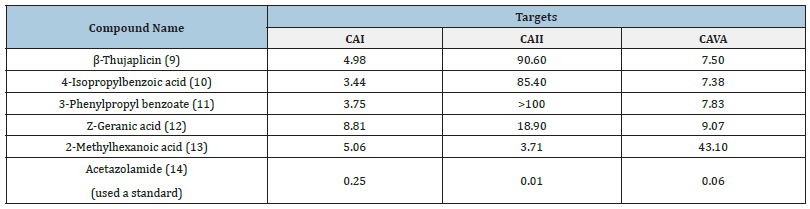
Figure 3:Structure of the CA inhibitors isolated form the essential oils as potential anti-obesity drugs.

Safety, Selectivity and Potential Side Effects of DGTA1 and Carbonic Anhydrase Inhibitors
Many effective weight-loss drugs have been pulled off the market because of potentially fatal adverse effects. These medications include phenylpropanolamine (stroke), fenfluramine (cardiac valvulopathy), dexfenfluramine (valvulopathy), aminorex (pulmonary hypertension), rimonabant (suicidal ideation and behavior), sibutramine (myocardial infarction and stroke), and the most recent medication, lorcaserin (cancer). Following the sibutramine withdrawal in 2010, the FDA requested cardiovascular safety information for new anti-obesity medications. Clinical trial results and novel drug development for effective anti-obesity medications must be carefully analyzed in light of potential safety issues and side effects, bearing in mind that obese people may regularly consume them [43,44]. Similarly, how topiramate in combination with phentermine was approved by the FDA in 2012 for a second use in the management of obesity, clinical trials are currently being conducted on the use of zonisamide, either alone or in combination with other medications (bupropion, metformin), for a similar purpose. However, although being efficacious, these pharmaceuticals are rarely employed as antiobesity therapies because of the side effects brought on by the non-selective inhibition of the target carbonic anhydrase enzyme isoforms and the polypharmacology of these medications [45].
Conclusion
In brief, these findings demonstrate that both Diacylglycerol O-Acyltransferase 1 (DGAT1) and carbonic anhydrase enzymes play vital roles in obesity and diabetes. The inhibitors of DGAT 1 and carbonic anhydrase have significant implications and are promising therapeutic approaches for the management of obesity and diabetes. Moreover, effective anti-obesity and anti-diabetic inhibitors must be prudently evaluated in light of potential safety issues and side effects.
Acknowledgment
We are grateful to TRC, Oman for providing a partial grant under project reference (ID# BFP/RGP/HSS/18./001).
References
- Sun H, Saeedi P, Karuranga S, Pinkepank M, Dianna J, et al. (2022) IDF diabetes atlas: Global, regional, and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183.
- Hussain H, Ivan RG, Abbas G, Sergazy MA, Hussain W, et al. (2019) Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: patent review (2015-2018). Expert Opinion on Therapeutic Patents 29(9).
- Abbas G, Harrasi AA, Hussain H, Hamaed A, Supuran CT (2019) The management of diabetes mellitus-imperative role of natural products against dipeptidyl peptidase-4, α-glucosidase and sodium-dependent glucose co-transporter 2 (SGLT2). Bioorganic Chemistry 86: 305-315.
- Torgerson JS, Hauptman J, Boldrin MN, Sjostr L (2004) XENical in the prevention of diabetes in obese subjects (XENDOS) study: A randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27(1): 155-161.
- Roux CW, Astrup A, Fujioka K, Frank G, David CW, et al. (2017) 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: A randomised, double-blind trial. SCALE Obesity Prediabetes NN8022-1839 Study Group 389(10077): 1399-1409.
- Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K (2002) The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 25(3): 608-613.
- Jackness C, Karmally W, Febres G, Irene MC, Leaque A, et al. (2013) Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and b-cell function in type 2 diabetic patients. Diabetes 62(9): 3027-3032.
- Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH (2014) Very-low-energy diet for type 2 diabetes: An underutilized therapy. Diabetes Complications 28(4): 506-510.
- O’Neil PM, Smith SR, Weissman NJ, Meredith CF, Sanchez M, et al. (2012) Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: The BLOOM-DM study. Obesity (Silver Spring) 20(7): 1426-1436.
- Ghulam A, Sulaiman AH, Ahmed AH, René C, Issa AA, et al. (2023) 11-Keto-β-Boswellic acid and 5-Chloro-8-hydroxyquinoline attenuate renal damage in streptozotocin-induced diabetic mice. Interventions Obes Diabetes 6(2): 554-558.
- Cases S (1998) Cloning of DGAT1, a novel mammalian diacylglycerol acyltransferase gene from intestine and adipose tissue. J Biol Chem 273(17): 10689-10697.
- Smith SJ, Cases S, Jensen DR, Hubert CC, Sande E, et al. (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87-90.
- Stone SJ, Levin MC, Farese RV (2006) Membrane topology and identification of key functional amino acid residues of murine acyl-CoA:diacylglycerol acyltransferase-2. J Biol Chem 281(52): 40273-40282.
- Villanueva CJ, Monetti M, Shih M, Ping Z, Steve MW, et al. (2009) Specific role for acyl CoA:diacylglycerol acyltransferase 1 (DGAT1) in hepatic steatosis due to exogenous fatty acids. Hepatology 50(2): 434-442.
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49(11): 2283-2301.
- Stone SJ, Myers HM, Watkins SM, Barbara EB, Kenneth RF, et al. (2004) Lipopenia and skin barrier abnormalities in DGAT1-deficient mice. J Biol Chem 279(12): 11767-11776.
- Bauman DE, Harvatine KJ, Lock AL (2011) Nutrigenomics, rumen-derived bioactive fatty acids, and the regulation of milk fat synthesis. Annu Rev Nutr 31: 299-319.
- Loomba R, Sanyal AJ (2013) The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 10(11): 686-690.
- Toke NH, Szkudlarek MM, Klaerke DA, Nielsen CH (2018) DGAT1 in lipid metabolism and diseases. Prostaglandins Other Lipid Mediat 139: 10-20.
- Bauman DE, Lock AL (2004) Concepts and issues in manipulating milk fat composition. J Dairy Sci 87(9): 405-411.
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV (2008) Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49(11): 2283-2301.
- Villanueva CJ, Monetti M, Shih M, Zhou P, Watkins SM, et al. (2009) Specific role for acyl CoA: diacylglycerol acyltransferase 1 (DGAT1) in hepatic steatosis due to exogenous fatty acids. Hepatology 50(2): 434-442.
- Smith SJ, Cases S, Jensen DR, Hubert CC, Eric S, et al. (2000) Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet 25: 87-90.
- Shao W, Espenshade PJ (2012) Expanding roles for SREBP in metabolism. Cell Metab 16(4): 414-419.
- Qi Y, Zhang Z, Zhao Y (2015) A genetic variant in the promoter region of miR-106b-25 cluster and risk of HBV infection and hepatocellular carcinoma. PLoS One 10(5): e0125830.
- Rong X, Wang B, Dunham MM, Niklas H, Jinny SW, et al. (2015) Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife 4: e06557.
- Yen CL, Stone SJ, Cases S (2008) DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res 49(11): 2283-2301.
- Cao J, Zhou Y, Peng H, Huang X, Stahler S, et al. (2011) Targeting acyl-CoA:diacylglycerol acyltransferase 1 (DGAT1) with small molecule inhibitors for the treatment of metabolic diseases. J Biol Chem 286(48): 41838-41851.
- Wu X, Huang Y, Chen Y (2011) Association of DGAT1 and LEP gene polymorphisms with intramuscular fat content in Yorkshire pigs. J Anim Sci 89(6): 1782-1789.
- Zhang J, Yang Q (2015) Diacylglycerol O-acyltransferase 1: a key mediator of hepatic steatosis and insulin resistance. World J Gastroenterol 21(28): 8026-8035.
- Tschapalda K, Zhang YQ, Liu L, Golovnina K, Schlemper T, et al. (2016) A class of diacylglycerol acyltransferase 1 inhibitors identified by a combination of phenotypic high-throughput screening, genomics, and genetics. EbioMedicine 8: 49-59.
- Huang JS, Guo BB, Wang GH, Zeng LM, Hu YH, et al. (2020) DGAT1 inhibitors protect pancreatic β-cells from palmitic acid-induced apoptosis. Acta Pharmacologica Sinica 42: 264-271.
- Wang H, Zhang J, Wu Q (2017) Carbonic anhydrase IX regulates adipogenic differentiation of human adipose-derived stem cells through the Wnt/β-catenin signaling pathway. Sci Rep 7: 43463.
- De Simone G, Supuran CT, Anna DF (2008) Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs 13(2): 383-392.
- Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7(2): 168-181.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT (2004) Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 19(3): 199-229.
- Nishimori I, Onishi S, Takeuchi H, Supuran CT (2010) Carbonic anhydrase inhibitors as potential therapeutic agents for gastric and pancreatic cancers. Expert Opin Ther Targets 14(2): 131-144.
- Andrea A, Mariana P, Stelian SM, Bogdan CS, Andrea M, et al. (2020) Supuran: Evaluation of thio- and seleno-acetamides bearing benzenesulfonamide as inhibitor of carbonic anhydrases from different pathogenic bacteria. International Journal of Molecular Sciences 21(2): 598.
- Andrea A, Ghulam A, Sonia P, Clemente C, Claudiu T (2018) Supuran: Selenides bearing benzenesulfonamide show potent inhibition activity against carbonic anhydrases from pathogenic bacteria vibrio cholerae and burkholderia pseudomallei. Bioorganic Chemistry 79: 319-322.
- Avvaru BS, Kim CU, Sippel KH, Sol MG, Mavis AM, et al. (2010) A short, strong hydrogen bond in the active site of human carbonic anhydrase II. Biochemistry 49(2): 249-251.
- Pucci L, Martinelli C, Ciofani G (2019) Carbonic anhydrase III: A neglected player in the pathogenesis of obesity and type 2 diabetes? Int J Mol Sci 20(9): 2091.
- Costa G, Gidaro MC, Vullo D, Supuran CT, Alcaro S (2016) Active components of essential oils as anti-obesity potential drugs investigated by in silico techniques. Journal of Agricultural and Food Chemistry 64(26): 5295-5300.
- Tak YJ, Lee SY (2021) Long-term efficacy and safety of anti-obesity treatment: where do we stand?. Current Obesity Reports 10: 14-30.
- Martins IJ (2016) Drug therapy for obesity with anti-aging genes modification. Annals of Obesity and Disorders 1(1): 1001.
- Supuran CT (2022) Anti-obesity carbonic anhydrase inhibitors: challenges and opportunities. Journal of Enzyme Inhibition and Medicinal Chemistry 37(1): 2478-2488.
© 2023 Ghulam Abbas and Quazi M I Haq. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






