- Submissions

Full Text
Intervention in Obesity & Diabetes
11-Keto-β-Boswellic Acid and 5-Chloro-8-Hydroxyquinoline Attenuate Renal Damage in Streptozotocin-Induced Diabetic Mice
Ghulam Abbas1,2*, Sulaiman Al-Hashmi2*, Ahmed Al-Harrasi2*, René Csuk3, Issa Al-Amri1,4, Najeeb Ur Rehman2, Ahmed Al-Adawi1 and Samera Khalaf4
1Department of Biological Sciences and Chemistry, University of Nizwa, Sultanate of Oman, Oman
2Natural and Medical Sciences Research Center, University of Nizwa, Sultanate of Oman, Oman
3Organic Chemistry, Martin-Luther University Halle-Wittenberg, Germany
4DARIS Research Center, University of Nizwa, Sultanate of Oman, Oman
*Corresponding author:Ghulam Abbas, Sulaiman Al-Hashmi and Ahmed Al- Harrasi, Department of Biological Sciences and Chemistry, Natural and Medical Sciences Research Center, University of Nizwa, PC 616, Nizwa, Sultanate of Oman
Emails: abbashej@unizwa.edu.om
sahashmi@unizwa.edu.om
aharrasi@unizwa.edu.om
Submission:February 06, 2023;Published: February 17, 2023

ISSN 2578-0263Volume6 Issue2
Abstract
The process of protein glycation is one of the major causes of diabetic nephropathy, which severely destroys the glomeruli within the kidney and is the leading cause of death in diabetic patients. The goal of this work was to assess the antiglycation and antioxidant capacity of 11-Keto-β-Boswellic Acid (KBA) and 5-Chloro-8-Hydroxyquinoline (CHQ) in vitro, as well as to investigate kidney damage in streptozotocininduced diabetic mice and investigate their probable mechanisms of action. CHQ showed a significant antiglycation effect in the antiglycation assay, with an IC50 value of <50μM, whereas KBA demonstrated good activity with an IC50=146.662.5μM when compared to rutin (IC50=98.01 2.03M) employed as a standard antiglycation agent. In 10–12-week-old CD1 mice, diabetes was induced by injecting 180mg/ kg of Streptozotocin (STZ) intraperitoneally. The normal mice group, the diabetic mice group, the KBA treated mice group, and the CHQ treated mice group were separated into four groups two weeks after STZ injection. The KBA and CHQ-treated mice received a 25mg/kg intraperitoneal dosage of KBA and CHQ, respectively. The blood glucose level (237.6 23.4mg/dl) measured after six weeks of STZ injection in the KBA treated mice group was significantly lower than the blood glucose level (415.8 25.2mg/dl) measured after two weeks. Histopathological changes in kidney structure confirmed that renal damage was reduced by reducing the enlargement of the mesangial matrix of the glomerulus in the nephron and significantly restoring Bowman’s gap in KBA and CHQ treated mice. The hypoglycemic action of KBA and antiglycation activity of CHQ can be linked with reduced kidney damage.
Keywords:Diabetic nephropathy; 11-Keto-β-boswellic acid; 5-Chloro-8-hydroxyquinoline; Streptozotocin-induced diabetic mice; Histopathology; Antiglycation; Antioxidant
Abbreviations:KBA: 11-Keto-β-Boswellic Acid; CHQ: 5-Chloro-8-Hydroxyquinoline; ROS: Reactive Oxygen Species; BSA: Bovine Serum Albumin; GFR: Glomerular Filtration Rate
Introduction
Protein glycation is one of the major causes of diabetes complications in diabetic patients, causing damage to all important organs, including the kidneys [1,2]. The body’s natural scavenging systems for free radicals are impaired as a result of diabetes, leading to free radical buildup and tissue damage. Reactive Oxygen Species (ROS) play an important role in the pathophysiology of diabetic nephropathy. Diabetic nephropathy is defined by the thickening of the glomerular basement membrane due to the expansion of the mesangial matrix, which is linked to glomerular filtration efficiency [3,4]. About 30 to 40 percent of diabetic patients suffer from diabetic nephropathy [5]. Diabetes is the major cause of diabetic nephropathy in Oman, according to a study, with a prevalence of 42.5 percent. It poses a serious threat to the health-care system. To control diabetes in the region, effective therapeutic and prevention strategies are required [6]. Tight control of blood glucose levels minimizes the risk of developing nephropathy. However, it is hard to achieve due to the limitations of available drug therapy. Hence, there is an urgent need for novel therapeutic agents in the quest for a more specific therapy [7,8]. Natural products extracted from medicinally important plants are under extensive investigation as they are rich sources of diverse biologically active and non-toxic compounds against different chronic diseases. A series of pentacyclic triterpene molecules (Boswellic acids) isolated from the gum resin of Boswellia serrata and Boswellia carteri have shown significant efficacy against various chronic diseases, including diabetes mellitus [9]. Azemi and co-workers revealed that the extract of B. serrata has antidiabetic properties [10]. The blood glucose, HbA1c, and lipid markers improved in two clinical trials involving type-2 diabetes patients who received Boswellia serrata resin [11]. The herbal formulation containing B. serrata Roxb. ex Colebr gum-resin also exhibited significant anti-diabetic activity [12].
The plant Boswellia sacra Flückiger, belongs to the Burseraceae family, which is widespread in the Dhofar region of Oman. Frankincense, an aromatic resin derived from boswellia trees, has a wide range of medical applications. Several studies have shown that the boswellia tree and its sticky resin are useful to a variety of ailments, including diabetes mellitus [13-15]. Previously, 8-Hydroxyquinoline (8HQ) and its derivatives exhibited wide medicinal properties, including antidiabetic, anticancer, antimicrobial, and antioxidant activities [16]. Quinoline derivatives were found to have significant antiglycation and antioxidant effects in vitro while being non-toxic in another investigation [17]. We investigated the effects of 11-keto-β-boswellic acid and 5-chloro- 8-hydroxyquinoline on protein glycation and diabetic nephropathy based on these findings. Our research group has previously worked on the isolation and characterisation of secondary metabolites from Boswella sacra, as well as the synthesis of numerous boswellic acid derivatives and their evaluation against a variety of biological disorders [18-21]. To examine antiglycation potential in vitro and in vivo mice models, we extracted 11-keto-β-boswellic acid from Boswella sacra and synthesized it on a large scale from boswellic acid [22,23], whereas 5-chloro-8-hydroxyquinoline was obtained from our in-house compound bank.
Materials and Methods
In-vitro studies
BSA-fluorescence assay: With minor adjustments, the same assay procedure as described earlier by Matsuda [24] and Matsuura [25] was used in this investigation. In a 100mM phosphate buffer with a pH of 7, Bovine Serum Albumin (BSA) solution (10mg/ mL) and anhydrous glucose 50mg/mL solution were prepared. The comparison of fluorescence intensity at 360nm excitations and emission at 440nm was obtained by using spectrofluorimeter. Rutin was used as standard inhibitor in this assay [26].
a. Statistical analysis:The results were expressed as
mean±SEM while the EZ-fit software (Perrella Scientific Inc.,
Amherst, U.S.A.) was used to calculate the IC50 values (μg/mL).
IC50 values were measured by using different concentrations of
the active samples.
% inhibition=100−(OD sample/OD Control) 100
DPPH-radical scavenging assay:Free radical scavenging activity of the compounds was determined by measuring the change in absorbance of DPPH (l,l-Diphenyl-2-picrylhydrazyl radical) by the spectrophotometric method described by Lee SK & co-workers [27]. The absorption was measured at 515nm by using spectrophotometers (Molecular Devices, CA, USA). The control contained 5μL of DMSO, instead of the test compound. The reactions were performed in triplicates.
In Vivo studies
Experimental animals:A total of 24 female CD1 mice, ranging in age from 10 to 12 weeks and weighing between 25 and 30g, were used in this study. Mice were housed in standard animal cages in a controlled environment (temperature 22±2 °C) fed with standard laboratory diet and water ad libitum. In order to adopt new conditions, mice were kept for one week in their new environment before starting the experiment. To ensure hygiene and maximum comfort animal’s bedding was changed twice a week. All experiments were approved by the university animal ethics committee for animal research.
Experimental design:The mice were first separated into two groups: a healthy control group (6 mice) and a streptozotocininduced group (18 mice). Six mice were kept in each cage. The mice in the control group (group 1) were injected with citrate buffer while the 18 mice in group 2 were Injected Intraperitoneal (IP) with streptozotocin (180mg/Kg) body weight in 10mM citrate buffer (pH 4.5). Two weeks following injection with streptozotocin, blood samples were collected from the mice’s tail vein. Streptozotocin induced mice with a blood glucose level higher than 300mg/dL were classified as diabetic mice and were further divided into three experimental groups, each containing 6 mice. Diabetic control mice (group 2) received water only, while group 3, and group 4 diabetic mice were treated with 11- keto-β-boswellic acid and 5-chloro-8- hydroxyquinoline dissolved in water at doses of 25mg/kg per day, respectively. Four weeks later, mice were first anesthetized, and blood was collected from the orbital vein. After that, mice were sacrificed and both kidneys were harvested for different analysis. The right kidney of each mouse was preserved in 4% formalin for histological studies, while the left kidney was snapped frozen in liquid nitrogen.
Renal histology studies:Kidney tissues from the cortical region of different mouse groups were stained with Hematoxylin and Eosin Stain (H & E) to show structural alterations in the kidney nephrons in this investigation. This research employed a Euromex Oxion Digital Light Microscope
Results and Discussion
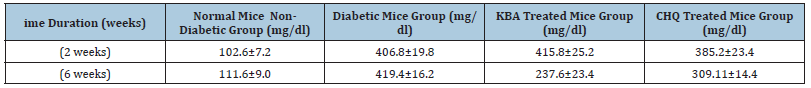
We investigated the inhibitory ability of 11-keto-β-boswellic acid and 5-chloro-8-hydroxyquinoline (Figure 1) against protein glycation in vitro and in vivo for the management of diabetes complications. We also examined the free radical scavenging (antioxidant) capacity of these samples because oxidative stress, free radical production, and diabetes are all linked. Due to its intriguing pharmacological effects, 11-keto-β-boswellic acid, a strong antiinflammatory molecule discovered from frankincense, has extended interest in recent decades [18,22,28]. KBA and CHQ demonstrated promising antilgycation activity in vitro with IC50 values of 146.661.5μM and <50μM, respectively, in a BSA-fluorescent based antiglycation test (Figure 2). This encouraged us to move forward with in vivo research. In the DPPH-radical scavenging (antioxidant) assay, CHQ exhibited moderate antioxidant activity with an IC50 value of 521.41±2.0μM while KBA remained inactive in this assay. Rutin and propyl gallate were used as standards in antiglycation and antioxidant assays respectively, as shown in Table 1. In this in vivo study, blood glucose levels of experimental mice groups were measured after two weeks of STZ injection to confirm that diabetes mellitus was induced and established in the target group. The blood glucose levels were measured again on the final day (after 6 weeks) just before sacrificing the animals. The blood glucose was found to be considerably lower in mice groups treated with KBA where an obvious difference was observed as 237.6±23.4mg/dl (after six weeks) as compared with 415.8±25.2mg/dl (after 2 weeks), while CHQ also moderately lowered the glucose level as 309.11±14.4mg/ dl (after six weeks) compared to 385.2±23.4mg. Conversely, there was no evident change in the blood glucose levels in the diabetic mice group with the passage of time, as shown in Table 2. Kidney tissues were collected from the cortical region and stained with Hematoxylin and Eosin Stain (H&E) to show nephron/s in the current investigation (Figure 3). Mesangial expansion, which is linked to Glomerular Filtration Rate (GFR), proteinuria, and hypertension, is the most visible structural change in diabetic nephropathy [29].
Figure 1: Chemical structures of 11-keto-β-boswellic acid and 5-chloro-8-hydroxyquinoline.
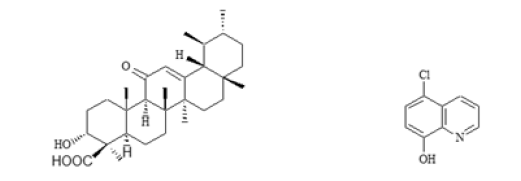
Figure 2: Dose-dependent antiglycation activity of KBA (■) and CHQ (▼) in vitro.
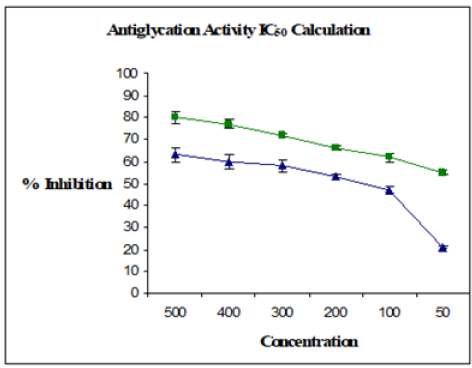
Figure 3:Renal histology images of mice from different experimental groups.
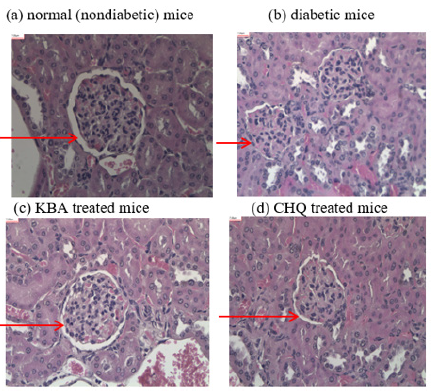
Table 1: Antiglycation and antioxidant activities in vitro. *NA=Not Active.
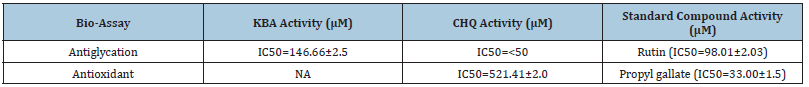
Table 2: Blood glucose levels in different mice groups.
One nephron is seen in the Hematoxylin and Eosin Stain (H&E) image of non-diabetic kidney tissue (a) (Figure 3). The renal corpuscle (glomerulus and bowman capsule) and convoluted tubules make up the kidney nephron. The morphology of the nephron is normal. The capillary tuft and mesangium in the Glomerulus (G) are normal. The Bowman Capsule (BC) that surrounds the glomerulus is in good condition. Tubules that were Proximal Convoluted (PC) and Distal Convoluted (DC) appeared to be normal. Two nephrons can be seen in the H&E image of diabetic kidney tissue (b). The morphology of the nephrons appeared to be aberrant. The mesangial matrix expansion in the Glomeruli (G) is evident. Due to mesangial matrix expansion, areas of Bowman space are obstructed. The convoluted tubules at the Proximal (PC) and Distal (DC) ends appeared normal. One nephron is visible in the H&E picture of renal tissue treated with KBA (c). When compared to diabetic kidney mesangial matrix expansion, it exhibits considerable recovery with little mesangial matrix expansion. The morphology of the nephron is normal. The capillary tuft and mesangium in the Glomeruli (G) are normal. The Bowman Capsule (BC) that surrounds the glomerulus is in good condition. Bowman Space (BS) is a standard size. Convoluted tubules Proximal (PC) and Distal (DC) appeared normal. Similarly, one nephron can be seen in the H&E image of mouse kidney tissue treated with CHQ (d). The morphology of diabetic mice’s nephrons improved after treatment with CHQ. When compared to diabetic glomerulus, the mesangial matrix of the glomerulus expended less and Bowmam’s space recovered partially. The results gleaned from the renal histology study revealed that KBA appreciably attenuated the mesangial matrix expansion in the glomeruli while CHQ showed moderate recovery of glomeruli expansion when compared to the diabetic kidney (control group) glomeruli mesangial expansion. Glycation of proteins is influenced by glucose levels, which could be the mechanism of action of these newly found anti-glycation agents. As a result, lesser kidney damage detected in the renal tissue in the KBA-treated mice group may be linked to the ability to manage blood glucose levels and hypoglycemic effects of KBA. CHQ’s in vivo efficacy can be explained by its anti-glycation and antioxidant characteristics, which are responsible for reducing kidney damage in the renal tissues of the CHQ-treated mice group.
Conclusion
In short, both 11-keto-β-boswellic acid and 5-chloro-8- hydroxyquinoline had strong antiglycation potential and thereby attenuated progression of diabetic nephropathy in Streptozotocin (STZ)-induced diabetic mice. In comparison to the diabetic mice group, histological examinations verified the reduced renal damage by lowering mesangial growth in the mice treated with KBA and CHQ. The mechanism-based analysis of these recently found antiglycation compounds revealed that KBA may minimize renal damage by reducing hyperglycemia and improving blood glucose levels, whilst CHQ’s antiglycation abilities may benefit renal damage.
Acknowledgment
We are grateful to Dr. Sireen A. Yaeesh for allowing us to use her lab facilities for some of our tests at the SQU, Muscat, Oman. We are also grateful to TRC, Oman for providing a partial grant under project reference (ID# BFP/RGP/HSS/18./001).
References
- Abbas G, Al-Harrasi AS, Hussain H, Hussain J, Rashid R, et al. (2016) Antiglycation therapy: Discovery of promising antiglycation agents for the management of diabetic complications. Pharm Biol 54(2): 198-206.
- Li Y, Tran VH, Duke CC, Roufogalis BD (2012) Preventive and protective properties of Zingiber officinale (ginger) in diabetes mellitus, diabetic complications, and associated lipid and other metabolic disorders: a brief review. Evid-Based Compl Alt.
- Sharma S, Anjaneyulu M, Kulkarni SK, Chopra K (2006) Resveratrol, a polyphenolic phytoalexin, attenuates diabetic nephropathy in rats. Pharmacology 76(2): 69-75.
- Punitha IR, Rajendran K, Shirwaikar A, Shirwaikar A (2005) Alcoholic stem extract of Coscinium fenestratum regulates carbohydrate metabolism and improves antioxidant status in streptozotocin–nicotinamide induced diabetic rats. Evid-Based Compl Alt 2(3): 375-381.
- Schena FP, Gesualdo L (2005) Pathogenetic mechanisms of diabetic nephropathy. J Am Soc Nephrol 16S: S30-S33.
- Alrawahi AH, Rizvi SG, Al-Riyami D, Al-Anqoodi Z (2012) Prevalence and risk factors of diabetic nephropathy in omani type 2 diabetics in Al-dakhiliyah region. Oman Med J 27(3): 212-216.
- Dalla Vestra M, Masiero A, Roiter AM, Saller A, Crepaldi G, et al. (2003) Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52(4): 1031-1035.
- Chan GC, Tang SC (2015) Diabetic nephropathy: landmark clinical trials and tribulations. Nephrol Dial Transpl 31(3): 359-368.
- Roy NK, Parama D, Banik K, Bordoloi D, Devi AK, et al. (2019) An update on pharmacological potential of boswellic acids against chronic diseases. Int J Mol Sci 20(17): 4101.
- Azemi ME, Namjoyan F, Khodayar MJ, Ahmadpour F, Padok AD, et al. (2012). The antioxidant capacity and anti-diabetic effect of Boswellia serrata triana and planch aqueous extract in fertile female diabetic rats and the possible effects on reproduction and histological changes in the liver and kidneys. Jundishapur J Nat Pharm Prod 7(4): 168-175.
- Ammon HPT (2019) Boswellic extracts and 11-keto-ß-boswellic acids prevent type 1 and type 2 diabetes mellitus by suppressing the expression of proinflammatory cytokines. Phytomedicine 63: 153002.
- Al-Awadi FA, Fatania HA, Shamte UM (1991) The effect of a plants mixture extract on liver gluconeogenesis in streptozotocin induced diabetic rats. Diabetes Res 18(4): 163-168.
- Abbas G, Albroumi MA, Rehman NU, Hussain H, Al-Harrasi AS (2017) Evaluation of essential oils from Boswellia sacra and Teucrium mascatense against acetyl cholinesterase enzyme and urease enzyme. Int J Phytomed 8: 500-505.
- Ni X, Suhail MM, Yang Q, Cao A, Fung KM, et al. (2012) Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complem Altern M 12: 253.
- Shehata AM, Quintanilla-Fend L, Bettio S, Singh CB, Ammon HP (2011) Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine 18(12): 1037-1044.
- Prachayasittikul V, Prachayasittikul S, Ruchirawat S, Prachayasittikul V (2013) 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des Devel Ther 7: 1157-1178.
- Bano B, Abbasi S, Khan JAJ, Hussain S, Rasheed S, et al. (2015) Antiglycation activity of quinoline derivatives- a new therapeutic class for the management of type 2 diabetes complications. Med Chem 11(1): 60-68.
- Al-Harrasi A, Ali L, Ceniviva E, Al-Rawahi A, Hussain J, et al. (2013) Antiglycation and antioxidant activities and HPTLC analysis of Boswellia sacra Oleogum resin: the sacred frankincense. Trop J Pharm Res 12(4): 597-602.
- Al-Harrasi A, Rehman NU, Mabood F, Albroumi M, Ali L, et al. (2017) Application of NIRS coupled with PLS regression as a rapid, non-destructive alternative method for quantification of KBA in Boswellia sacra. Spectrochem Acta A 184: 277-285.
- Csuk R, Barthel-Niesen A, Barthel A, Schäfer R, Al-Harrasi A (2015) 11-Keto-boswellic acid derived amides and monodesmosidic saponins induce apoptosis in breast and cervical cancers cells. Eur J Med Chem 100: 98-105.
- Csuk R, Niesen-Barthel A, Schäfer R, Barthel A, Al-Harrasi A (2015) Synthesis and antitumor activity of ring A modified 11-keto-β-boswellic acid derivatives. Eur J Med Chem 92: 700-711.
- Rehman NU, Khan A, Al-Harrasi A, Hussain H, Wadood A, et al. (2018) New α-glucosidase inhibitors from the resins of Boswellia species with structure–glucosidase activity and molecular docking studies. Bioorg Chem 79: 27-33.
- Shamraiz U, Hussain H, Rehman NU, Al-Shidhani S, Saeed A, et al. (2019) Synthesis of new boswellic acid derivatives as potential antiproliferative agents. Nat Prod Res 34(13): 1845-1852.
- Matsuda H, Wang T, Managi H, Yoshikawa M (2003) Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg Med Chem 11(24): 5317-5323.
- Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, et al. (2002) Screening system for the maillard reaction inhibitor from natural product extracts. J Health Sci 48: 520-526.
- Mosihuzzman M, Naheed S, Hareem S, Talib S, Abbas G, et al. (2013) Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life Sci 92(3): 187-192.
- Lee SK, Mbwambo ZH, Chung H, Luyengi L, Gamez EJ, et al. (1998) Evaluation of the antioxidant potential of natural products. Comb Chem High Throughput Screen 1(1): 35-46.
- Al Amri I, Mabood F, Kadim IT, Alkindi A, Al-Harrasi A, et al. (2021) Evaluation of the solubility of 11-keto-β-boswellic acid and its histological effect on the diabetic mice liver using a novel technique. Veterinary World 14(7): 1797-1803.
- Toth-Manikowski S, Atta MG (2015) Diabetic kidney disease: pathophysiology and therapeutic targets. J Diabetes Res.
© 2023 Ghulam Abbas, Sulaiman Al-Hashmi and Ahmed Al- Harras. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






