- Submissions

Full Text
Intervention in Obesity & Diabetes
Specific Anti-Obese Synbiotics to Suit Genetically Different Obese Persons
M Haridas* and A Sabu
Inter University Centre for Bioscience and Dept. Biotechnology & Microbiology, India
*Corresponding author: M Haridas, Inter University Centre for Bioscience and Dept. Biotechnology & Microbiology, Dr Janaki Ammal Campus, Kannur University, India
Submission:March 09, 2020;Published: April 16, 2020

ISSN 2578-0263Volume3 Issue5
Abstract
Ayurvedic fermentation as prescribed in classical texts is a versatile and powerful protocol for developing novel, traditional-like medicines. Ayurvedic medication, having a Prakriti (genetic) determinant, needs to be considered suitable for addressing metabolic disorders with immunologic implications, influenced by gut microbiome (as evidenced by the enterotypes). Obesity is a pathologic state on which the gut microbiota exercises influence in inflammatory and metabolic ways. When coupled with reverse pharmacology it would yield highly predictable results. It also bears potentials for redefining the protocol itself to deliver future products. In this article we essay on conceptualising the production and the prospect of developing new anti-obesity fermented nutraceutics/synbiotics to suit the prakriti of obese persons by prakriti-specific gut microbiome.
Keywords: Selenium; Diabetes; Obesity; Insulin resistance; Immune regulation; Gut microbiota
Introduction
Gut microbiota is likely to be one of the factors influencing our predisposition to develop obesity and associated morbidities. Alterations in the gut microbiota structure have been related to obesity [1-4]. Gut microbiota is likely to be involved in body weight regulation by influencing the host’s metabolic and endocrine network, and as a consequence of diet and other environmental factors [3-5] and of the genotype [1,2]. The same genotype under the same dietary influence of high-fat diet (HFD) developed different metabolic phenotypes as a function of their specific gut microbiota for developing metabolic dysfunction [6], depending on gut microbiota [7]. Therefore, a growing body of scientific evidence supports the notion that the crosstalk between the gut microbiota, diet and immune system activates mediators and signaling pathways, for pathologic convergence to metabolic diseases and obesity. The innate immune system is one of the key regulators of the crosstalk between the host and the microbiota (commensal and pathogenic microbes) and it is associated with immunological dysfunctions in relation to obesity and metabolic diseases. Interactions between the gut microbiota and immune responses are found to be very complex [8]. Furthermore, it has been evidenced that intestinal inflammation is an early event preceding obesity as a metabolic disease. It could be altered by dietary-modulation and by the gut microbiota through intervention strategies, designed to combat such disorders. In this context, it is essential to identify the exact process. The caloric content and composition of diets are linked to the development of overweight and obesity. It is seen associated with major shifts in the gut microbiota, both in laboratory animals and in human beings [9]. Latest estimates of the cultured fraction of sequence-based diversity of gut microbiota range between 35% and 65% [10] and the multitude of strains and experimental approaches used [11].
In this context, we find two things are important in tackling the issue of obesity, the contents of the food and gut microbiota of the person. Low calorific and low fat food intake is advised for quite some time together with antilipidemic agents or antihyperlipidemic drugs. However, there is no conceptual method for manipulating food to suit obesity either. Fecal microbiota transplantation (FMT) is an efficient way to test causal effects of changes in microbial diversity and composition on pathophysiological parameters. FMT has been applied in a variety of pathologies, including obesity [10]. Implementation of functional food (prebiotics and synbiotics) in the clinics for targeted manipulation of the gut microbiota may prove to be helpful [12,13]. It is hypothesized that the gut microbiota influences intestinal fatty acid absorption, via for instance, modulation of the luminal bile acid pool or the expression of transporters. The lipids, such as short-chain fatty acids, bile acids, phosphatidylcholine, conjugated fatty acids, and hydroxy fatty acids play a role in microbe-host interactions and are thought to be relevant for microbe-host communication. It seems, the gut microbiota is not the primary cause of obesity, however, much need to be studied. Constituents of diet are metabolized by the gut microbiome as well, to elicit beneficial or harmful metabolites. By combining 22 newly sequenced faecal metagenomes of individuals from four countries with previously published data sets, three robust clusters were identified, based on the prevalence of Prevotella, Bacteroides, or Ruminococcus in their gut microbiome (referred to as enterotypes hereafter) that are not nation or continent specific [14]. The enterotypes are mostly driven by species composition. However, large amount of molecular functions are not necessarily provided by the largely present species, highlighting the importance of a functional analysis to understand microbial communities. It is argued that individual host properties such as body mass index, age, or gender cannot explain the observed enterotypes. The data-driven marker genes or functional modules can be identified for each of these host properties. For example, twelve genes significantly correlate with age and three functional modules with the body mass index, hinting at a diagnostic potential of microbial markers [14].
The ancient Indian system of medicine Ayurveda distinguishes humans into three types based on human nature (genotypes or ‘Prakritis’). Genome-wide analysis done by Govindaraj et al. [15] correlated the three ‘Ayurveda Prakritis’ (Ayurvedic genotypes), as shown below in Figure 1. So far no successful attempt has been made to correlate specifically the three enterotypes and the three Ayurveda Prakritis have anything in common or one specific enterotype would correspond to an Ayurvedic genotype (Ayurveda Prakriti) across the humanity. However, the enterotypes are also have a genetic basis or correlated genetically. The method of Ayurvedic medication, having a Prakriti (genetic) determinant, needs to be considered suitable for addressing metabolic disorders with immunologic implications influenced by gut microbiome (as evidenced by the enterotypes). Obesity is a disease on which the gut microbiota exercises influence in inflammatory and metabolic ways.
Figure 1:
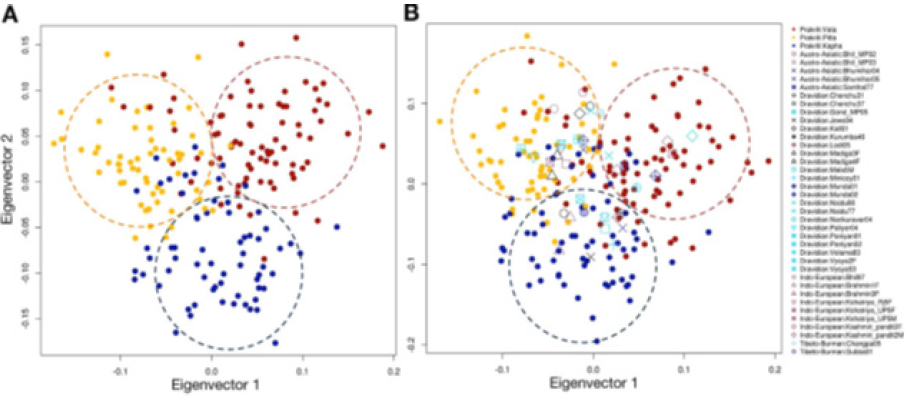
Fermentation is a prescribed method of drug preparation in Ayurveda, with proven enhanced therapeutic properties [16]. A potential application of a fermented nutraceutical (fermented papaya preparation) in acute respiratory illnesses has been demonstrated. An in-vivo placebo-controlled, cross-over clinical study in different age groups of healthy subjects, has shown better activity of biotansformed secondary metabolites [17,18]. Heo et al. [19] showed by their recent studies on gut microbiota that obesogenic bacteria exacerbate obesity and metabolic dysfunction in the host when fed a high fat diet (HFD). Also, there are several studies showing effectiveness of fermentation to make better nutracuetical/medicinal (Ayurvedic) products [16,20-25]. Dietary intervention with probiotics and nutraceuticals fermented with healthy gut microbiota of specific genotype (Ayurveda Prakriti) of the obese person would be an effective method of containing obesity. Hence, altogether three streams of products made by using three distinct healthy enterotypes only may be required to suit the entire humanity divided into three types based on the genetic nature.
Application of Synbiotics
It refers to food ingredients or dietary supplements combining probiotics and prebiotics in a form of synergism, hence it may be called synbiotics. The synbiotic concept was first introduced as ‘mixtures of probiotics and prebiotics’ that beneficially affect the host by improving the survival and implantation of live microbial community and dietary supplements in the gastrointestinal tract. (The Ayurvedic fermentation is extremely prolonged compared to alcoholic fermentation and would effectively produce synbiotics within them.) By selectively stimulating the growth and/or by activating the metabolism of one or a limited number of healthpromoting bacteria may be the real starter of a synbiotic product and a major breakthrough may be expected in future.
Outcome
Gut microbiota is likely to be one of the factors influencing our predisposition to develop obesity and associated co-morbidities. A growing body of scientific evidence supports the notion that there exists crosstalk between the gut microbiota, diet and immune system activating mediators and signaling pathways and found to be very complex. It is hypothesized that the gut microbiota influences intestinal fatty acid absorption, via, for instance, modulation of the luminal bile acid pool or the expression of transporters. Faecal microbiota transplantation (FMT) has been applied in a variety of pathologies, including inflammatory bowel diseases and obesity. Functional food (prebiotics and synbiotics of Ayurvedic model) in the clinics for targeted manipulation of the gut microbiota may prove to be helpful.
References
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444 (7122): 1022-1023.
- Waldram A, Holmes E, Wang Y, Rantalainen M, Wilson ID, et al. (2009) Top-down systems biology modeling of host metabotypemicrobiome associations in obese rodents. J Proteome Res 8(5): 2361-2375.
- Gauffin Cano PG, Santacruz A, Trejo FM, Sanz Y (2013) Bifidobacterium CECT 7765 improves metabolic and immunological alterations associated with obesity in high-fat diet-fed mice. Obesity (Silver Spring) 21(11): 2310-2321.
- Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, et al. (2013) Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341(6150): 1241214.
- Burcelin R, Crivelli V, Dacosta A, Roy Tirelli A, Thorens B (2002) Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab 282(4): E834-E842.
- Serino M, Luche E, Gres S, Baylac A, Berge M, et al. (2012) Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 61(4): 543-553.
- Sanz Y, De Palma G (2009) Gut microbiota and probiotics in modulation of epithelium and gut-associated lymphoid tissue function. Int Rev Immunol 28(6): 397-413.
- Zamora N, Bashiardes S, Levy M, Elinav E (2017) The role of the immune system in metabolic health and disease. Cell Metab 25(3): 506-521.
- Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, et al. (2014) High-fat diet alters gut microbiota physiology in mice. The ISME Journal 8(2): 295-308.
- Paramsothy S, Kamm MA, Kaakoush NO, Walsh AJ, Van den Bogaerde J, et al. (2017) Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: A randomised placebo-controlled trial. Lancet 389(10075): 1218-1228.
- Kumar M, Nagpal R, Kumar R, Hemalatha R, Verma V, et al. (2012) Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Experimental Diabetes Research 2012: 902917.
- Ferolla SM, Couto CA, Costa Silva L, Armiliato GN, Pereira CA, et al. (2016) Beneficial effect of synbiotic supplementation on hepatic steatosis and anthropometric parameters, but not on gut permeability in a population with non-alcoholic steatohepatitis. Nutrients 8(7): E397.
- Lambert JE, Parnell JA, Eksteen B, Raman M, Bomhof MR, et al. (2015) Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: A randomized controlled trial protocol. BMC Gastroenterology 15: 169.
- Arumungam M, Raes J, Pelletier E, Le Paslier D, Yamada T, et al. (2011) Enterotypes of the human gut microbiome. Nature 473(7346): 174-180.
- Govindaraj P, Nizamuddin S, Sharath A, Jyothi V, Rotti H, et al. (2015) Genome-wide analysis correlates ayurveda prakriti. Scientific Reports 5: 15786.
- Chandra DN, Preethidan DS, Sabu A, Haridas M (2015) Traditional fermentation of Ayurvedic medicine yields higher proinflammatory enzyme inhibition compared to wine-model product. Frontiers in Life Science 8(2): 160-164.
- Marotta F, Naito Y, Jain S, Lorenzetti A, Soresi V, et al. (2012) Is there a potential application of a fermented nutraceutical in acute respiratory illnesses? An in-vivo placebo-controlled, cross-over clinical study in different age groups of healthy subjects. Journal of biological regulators and homeostatic agents 26(2): 285-294.
- Marotta F, Yadav H, Kumari A, Catanzaro R, Jain S, et al. (2012) Cardioprotective effect of a biofermented nutraceutical on endothelial function in healthy middle-aged subjects. Rejuvenation research 15(2): 178-181.
- Heo J, Seo M, Park H, Lee WK, Guan LL, et al. (2016) Gut microbiota modulated by probiotics and garcinia cambogia extract correlate with weight gainm and adipocyte sizes in high fat-fed mice. Scientific Reports 6: 33566.
- Sabu A, Haridas M (2015) Fermentation in ancient ayurveda: Its present implications. Frontiers in Life Science 8(4): 324-331.
- Chandra DN, Prasanth GK, Singh N, Kumar S, Jithesh O, et al. (2011) Identification of a novel and potent inhibitor of phospholipase A(2) in a medicinal plant: crystal structure at 1.93Å and surface plasmon resonance analysis of phospholipase A(2) complexed with berberine. Biochim Biophys Acta 1814(5): 657-663.
- Chandra DN, Abhilash J, Prasanth GK, Sabu A, Sadasivan C, et al. (2012) Inverted binding due to a minor structural change in berberine enhances its phospholipase A2 inhibitory effect. Int J Biol Macromol 50(3): 578-585.
- Tomy MJ, Sharanya CS, Dileep KV, Prasanth S, Sabu A, et al. (2015) Derivatives form better lipoxygenase inhibitors than piperine: in vitro and in silico study. Chem Biol Drug Des 85(6): 715-721.
- Tomy MJ, Sharanya CS, Mahapatra DK, Suresh KI, Sabu A, et al. (2018) In vitro assessment of selected benzoic acid derivatives as anti-inflammatory compounds. Journal of Scientific & Industrial Research 77: 77-83.
- Sharanya CS, Shabeer Ali H, Sabu A, Haridas M (2019) Fermentation of polyherbal preparations as in ayurveda: A novel protocol for drug-lead discovery. J Nat Ayurvedic Med 3(3): 000197.
© 2020 M Haridas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






