- Submissions

Full Text
Gerontology & Geriatrics Studies
Phase Angle as a Marker for Sarcopenia and Frailty in Hospitalized Older Adults
Nathália Carla de Andrade Pereira1, Roana Carolina Bezerra dos Santos1, Letícia Sabino Santos1, Taynara de Sousa Rego Mendes1, Stefany Beatriz do Nascimento1, Maria Conceição Chaves de Lemos2 and Cláudia Porto Sabino Pinho1*
1Clinics Hospital, Federal University of Pernambuco, Brazilian Company of Hospital Services, Brazil
2Department of Nutrition, Federal University of Pernambuco, Brazil
*Corresponding author:Claudia Porto Sabino Pinho, Hospital of Clinics, Federal University of Pernambuco, Brazilian Company of Hospital Services (EBSERH). Prof. Moraes Rego Av., N/N, University City, Recife, Pernambuco, Brazil
Submission: June 27, 2025; Published: July 28, 2025

ISSN 2578-0093Volume9 Issue 5
Abstract
Background: Previous studies have indicated that the phase angle (PhA) can serve as a valuable nutritional marker, yet limited research has explored its potential as a predictor for sarcopenia and frailty.
Objective: To assess the Phase Angle (PhA) as a marker for sarcopenia and frailty in hospitalized geriatric patients.
Methods: This cross-sectional study enrolled hospitalized individuals aged 60 years or older, of both sexes, admitted to a hospital in “city removed”. The PhA was determined through measurements of resistance and reactance obtained from electrical bioimpedance analysis. Sarcopenia was defined based on the European Working Group on Sarcopenia in Older People 2 criteria and frailty was assessed using Fried’s criteria.
Results: Our sample consisted of 176 patients with an average age of 69.8±7.8 years. Sarcopenia was found in 37.7% of our population, while frailty was found in 67.0%. Reduced PhA was found in 56.3% of the individuals. A low PhA was independently associated with sarcopenia [Adjusted OR: 2.7 (95% CI: 1.2-5.8] and frailty [Adjusted OR: 2.4 (95% CI: 1.1-5.2)]. Patients with severe sarcopenia (Median: 4.0; IQR: 3.0-5.0; p<0.001) and frailty (Median: 4.0; IQR: 3.0-5.0; p=0.001) exhibited significantly lower median PhA values.
Conclusion: The PhA can be regarded as a valuable marker for identifying sarcopenia and frailty in hospitalized geriatric patients. Additionally, lower PhA values may reflect the severity of sarcopenia.
Keywords:Nutritional assessment; Bioelectrical impedance analysis; Sarcopenia; Frailty; Aging
Introduction
The rise in life expectancy and the growing population of older individuals worldwide are contributing to a higher incidence of chronic diseases associated with aging, comorbidities and geriatric syndromes. This situation poses a significant and substantial challenge, both economically and for healthcare systems [1-6]. Among geriatric syndromes, sarcopenia and frailty stand out as frequent conditions linked to adverse outcomes and heightened morbidity and mortality risk. Sarcopenia is defined by a decrease in both muscle mass and function [4,5], whereas frailty is a multidimensional syndrome characterized by signs and symptoms that forecast increased susceptibility to stress-related events and unfavorable outcomes, including falls, fractures, hospitalization, disability and mortality [2,5,6]. Assessing the nutritional status and geriatric syndromes in hospitalized geriatric patients presents a significant challenge, especially considering that many of them are bedridden, have limited mobility, or are in critical medical conditions. Consequently, the choice of practical and applicable assessment tools that can be conveniently performed at the bedside and tailored to the patient’s condition becomes essential [3,7,8]. In this context, the phase angle (PhA) has emerged as a potentially valuable biomarker for sarcopenia and frailty in recent studies [9,10]. However, this association has not been extensively investigated and initial findings have been subject to debate and controversy [11-13]. PhA is a parameter derived from Bioelectrical Impedance Analysis (BIA) and it is defined as the arctangent of the ratio of resistance (R) to reactance (Xc) [14]. In simpler terms, PhA represents the angular shift (phase difference) that occurs when an electrical current interacts with a cell [15,16]. It is considered a valuable indicator of cellular health and membrane integrity. Lower PhA values are indicative of cell damage or a breakdown in the selective permeability of the cell membrane [17], while higher values suggest preserved cellular activity [14]. BIA is a straightforward and reproducible technique that employs a portable, non-invasive instrument. It can be applied at various stages of nutritional care and the European Consensus [3] has practically suggested that using BIA to calculate the PhA can serve as a strategy to assess overall muscle quality [3,11]. Given that the diagnosis of sarcopenia and frailty relies on criteria that demand preserved physical capacity, BIA can be a valuable tool in individuals who cannot perform manual grip strength tests or are unable to walk [18]. In this context, there is currently no consensus regarding the predictive capacity of the PhA as a useful marker for screening these geriatric syndromes. More research is needed to obtain more consistent results. Thus, our study aimed to evaluate the PhA as a potential marker for sarcopenia and frailty in hospitalized geriatric patients.
Methods
Study design and participants
This is a cross-sectional study involving individuals admitted for hospitalization between March and September 2021 at a university hospital in Recife-PE, Brazil. The hospital serves a wide spectrum of clinical and surgical specialties, including internal medicine, oncology, urology, gynecology, vascular surgery, orthopedics, general surgery, infectious parasitic diseases and nephrology. Patients of both sexes aged 60 years or older were included in the study. Individuals with physical and cognitive limitations, those under complete bed rest, patients in the immediate post-operative period of medium and large surgeries, individuals with mechanical prostheses, those with chronic kidney disease undergoing dialysis treatment and/or those with joint abnormalities in the upper and lower limbs that rendered them unable to perform the proposed tests were excluded. The sample size was determined using the STATCALC module in Epi Info® software, version 6.04. It took into account a 5% α error, a 20% β error, a correlation coefficient of 0.326 (p) between muscle strength and PA, as reported in a previous study with hospitalized older patients 1 and a variability of 0.1(d²). This calculation yielded a minimum sample size of 173 patients. To accommodate potential losses, an additional 10% was added to the minimum sample size, resulting in a final sample size of 191 older people.
Phase angle assessment
PhA was assessed through BIA, which involved obtaining measurements of Resistance (R) and Reactance (Xc) using the equation: Xc(Ω)/R(Ω) [19]. To convert the result into degrees, the obtained value was multiplied by 180/π15, as impedance values were obtained using the portable Biodynamics instrument model 310. This BIA device applies a current of 800μA at a single frequency of 50kHz. The measurements of R and Xc were recorded in Ohms [20,21]. Data collection was performed within 72 hours of hospital admission. Patients were provided with instructions to adhere to a preparation protocol before undergoing the BIA test. This protocol included guidelines such as refraining from caffeine consumption for 24 hours prior, emptying their bladder 30 minutes before the test, removing any potential metallic objects and avoiding substantial meals within 4 hours before the test. During the assessment, patients were positioned in a supine dorsal decubitus on a non-metallic surface, with the headrest parallel to the ground. Their arms were extended at an approximate angle of 30o away from the body and their legs were spaced apart at an approximate angle of 45o. Two electrodes were placed distally on the dorsal surface of the hand and foot, while another two electrodes were carefully positioned between the wrist and ankle, specifically at the medial and lateral malleoli. The cutoff point suggested by Rosas-Carrasco, et al. [9] was employed, with PhA≤4.1o classified as indicating a low value.
Sarcopenia assessment
Sarcopenia was determined based on the presence of both reduced muscle strength and mass. To assess the severity of sarcopenia, a physical performance evaluation was conducted using the gait speed test. Severe sarcopenia was defined when both reduced muscle strength and mass were present, along with slow gait speed [3]. Muscle strength was evaluated using the Hand Grip Strength (HGS) Test, following the standardized procedures recommended by the American Society of Hand Therapists [1,2]. A JAMAR® digital dynamometer was employed for the measurements. Participants were instructed to sit with their shoulders adducted and in a neutral position, their elbows flexed at 90°, their arms in a neutral position and their wrists between 0 and 30° of flexion and between 0 and 15° of ulnar deviation. A trained evaluator guided the participants through three consecutive measurements on their dominant hand, with a 15-second rest interval between each attempt, each lasting 5 seconds. The highest recorded value was considered and the results were recorded in kg/f. To establish a diagnosis of low muscle strength, cutoff points for HGS, as proposed in EWGSOP 2 [3], were used. Values of HGS <27kg/f for men and <16kg/f for women were considered indicative of low muscle strength. Appendicular Skeletal Muscle Mass (ASMM) was derived using the equation by Sergi et al. [22] SMM = (0.227* Resistance Index (RI)) + (0.064* Reactance (Xc)) +(0.095*weight(P)) +(1.384*sex)-3.964. The measurement of resistance was obtained through BIA, using the technique described above. From the result of the Sergi²² equation, the Appendicular Skeletal Muscle Mass Index (ASMI) was calculated using the formula: ASMM/Height² and classified according to the cutoff points suggested for the “nameremoved” population, where values ≤7.7kg/m² in men and ≤5.62 kg/m² in women are indicative of low muscle mass [23]. Physical performance was evaluated using the gait speed test, which involved measuring the time it took for the participant to walk a 4-meter distance. Each patient underwent the test twice and the faster of the two recorded speeds was used. Participants were instructed to walk at their typical pace. Gait speed was categorized as slow if it was <0.8 meters/second and considered adequate for values above this threshold3.
Frailty assessment
To evaluate frailty, the study analyzed the phenotypic criteria
proposed by Fried [5]. A participant was classified as frail if they
met three or more of the following criteria:
A. Unintentional weight loss
B. Self-reported exhaustion or fatigue
C. Reduced HGS
D. Low levels of physical activity
E. Reduced gait speed.
Unintentional weight loss (≥4.5 kg or ≥5% of body weight in the last 12 months) was calculated using the following equation: %Weight loss = (Usual Weight-Current weight) x100/Usual weight. Fatigue was assessed based on self-reported exhaustion, indicated by two questions from the Center for Epidemiological Studies-Depression (CES-D)scale: “Did you feel that you had to exert yourself to accomplish your usual activities?” and “Did you feel that you could not get going?”. If the individual responded with “most of the time and/or always” to either of these two questions, it was considered a criterion for frailty [24]. Muscle strength was evaluated using the technique and cutoff points previously mentioned. Low levels of physical activity were assessed using the International Physical Activity Questionnaire (IPAQ) in its short version. Individuals who engaged in less than 150 minutes of physical activity per week were classified as insufficiently active or sedentary and this was considered one of the criteria for frailty [25]. Physical performance was determined through the gait speed test, as previously explained.
Sociodemographic, clinical and nutritional data
The analysis took into account the following variables: Age, sex, self-reported race, clinical diagnosis upon admission (categorized as malignant and non-malignant disorders [26], the presence of comorbidities like Systemic Arterial Hypertension (SAH) and Diabetes Mellitus (DM), body mass index and Mini Nutritional Assessment (MNA). The Body Mass Index (BMI), calculated as the ratio of Weight (kg) to Height (m)², was categorized using the following thresholds: Underweight when BMI<22 kg/m², normal weight when BMI ranged from 22to27 kg/m² and overweight when BMI exceeded 27kg/m² [27]. The Mini Nutritional Assessment (MNA) categorized individuals as follows: normal nutritional status (score≥24), at risk of malnutrition (score between 17 and 23.5) and malnourished (score <17) [28].
Ethical aspects
The study adhered to ethical principles for research involving human participants, following the guidelines outlined in Resolution No. 466/2012 of the National Health Council. It underwent a thorough ethical review and received approval from the Research Ethics Committee for Human Research at the Clinics Hospital of Pernambuco under protocol number 4.579.177/2021. All participants were provided with detailed information about the research objectives and procedures and gave their informed consent by signing the Informed Consent Form.
Data analyses
We used the statistical program SPSS, version 13.0 (SPSS Inc., Chicago, IL, USA) to data analyses. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test and they were described as mean and standard deviation when they showed a normal distribution and as median and Inter Quartile Range (IQR) when they had a non-normal distribution. Univariate analysis was performed using the Chi-Square test. A binary logistic regression model was constructed to examine the factors associated with low PhA. Independent variables were tested for multicollinearity using collinearity statistics VIF (Variance Inflation Factor) (>0.10and<3) and Tolerance. Two regression models were developed, separating sarcopenia and frailty due to collinearity issues. Variables with a p-value <0.20 in the univariate analysis were included in the multivariate analysis. Adjusted odds ratios (OR) were estimated, along with their respective 95% confidence intervals using Wald’s test. The goodness of fit of the model was checked using the Hosmer-Lem show test. Analyses with a significance level <0.05 were considered significant for the final model.
Results
A total of 191 individuals were enrolled in our study, but 18 patients were excluded from the analysis due to data inconsistencies or a lack of BIA results. Consequently, the final sample comprised 176 participants. The average age was 69.8±7.8 years, with 54.5% being male. The majority of participants fell within the 60 to 69- year age bracket (56%) and a significant proportion identified as non-white (of mixed race or Black ethnicity) (63.6%). It was noted that 29.0% of the participants were underweight according to their BMI and 61.4% were at risk of malnutrition or undernutrition as indicated by the MAN assessment. The median PhA was 4.0° (IQR: 3.9°-5.0°), with 56.3% of participants exhibiting a low PhA (<4.1°). The prevalence of sarcopenia was 37.7% and frailty was observed in 67.0% of the individuals (Table 1). In our univariate analysis, PhA was not associated with demographic and clinical variables. However, PhA was associated with low BMI (p=0.031), malnutrition or risk of malnutrition according to the MAN assessment (p=0.027), as well as sarcopenia (p<0.001) and frailty (p=0.002) (Table 2). The adjusted analysis in the multivariate model showed that reduced PhA was independently associated with sarcopenia [Adj OR: 2.7 (95% CI 1.2-5.8)] and frailty [Adj OR: 2.4, (95% CI 1.1-5.2)]. Interestingly, nutritional status, as determined by BMI and the MAN assessment, ceased to be associated with a low phase angle after accounting for confounding variables (Table 3). When we compared the median PhA values with geriatric syndromes and their diagnostic components, some noteworthy findings emerged. Patients with sarcopenia (Median: 4.0°; IQR: 3.0°-5.0°; p<0.001) and frailty (Median: 4.0°; IQR: 3.0°-5.0°; p=0.001) exhibited lower PhA values, when compared to no sarcopenia or no frailty. Further stratification by the severity of sarcopenia revealed that those with severe sarcopenia had even lower median PhA values (Median: 4.0°; IQR: 3.0°-4.1°; p<0.001). In the analysis of PhA based on diagnostic components, it became evident that patients with reduced HGS had reduced PhA values (Median: 4.0°; IQR: 3.0°-5.0°; p<0.001), as did individuals with low muscle mass (ASMI) (Median: 4.0°; IQR: 3.0°-5.0°; p<0.001) and those who had experienced unintentional weight loss (Median: 4.0°; IQR: 3.0°-5.0°; p<0.003). However, no significant differences in median PhA were observed based on the presence of fatigue or in relation to levels of physical activity and physical performance (p>0.05) (Table 4).
Table 1:Demographic, clinical, nutritional characteristics and sarcopenia and frailty components in elderly hospitalized patients (n=176).
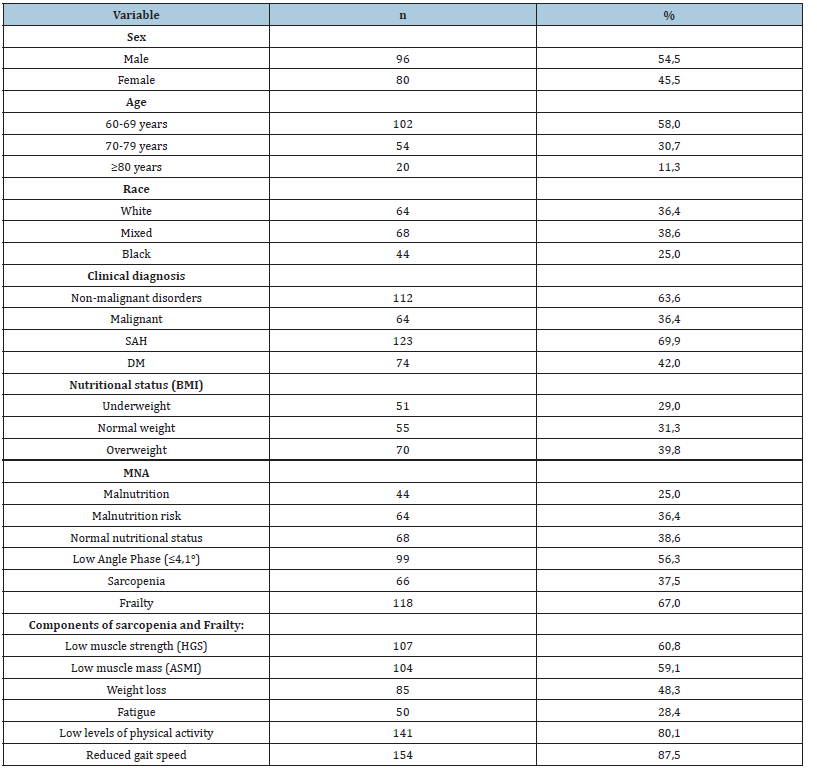
ASMI: Appendicular Skeletal Muscle Mass Index; BMI: Body Mass Index; DM: Diabetes Mellitus; HGS: Hand Grip Strength; MNA: Mini Nutritional Assessment; SAH: Systemic Arterial Hypertension. Sarcopenia defined by the European Sarcopenia Consensus (3); Frailty defined by Fried (5).
Table 2:Analysis of demographic, clinical, nutritional factors, sarcopenia and frailty associated with low Phase Angle in hospitalized elderly (n=176)..
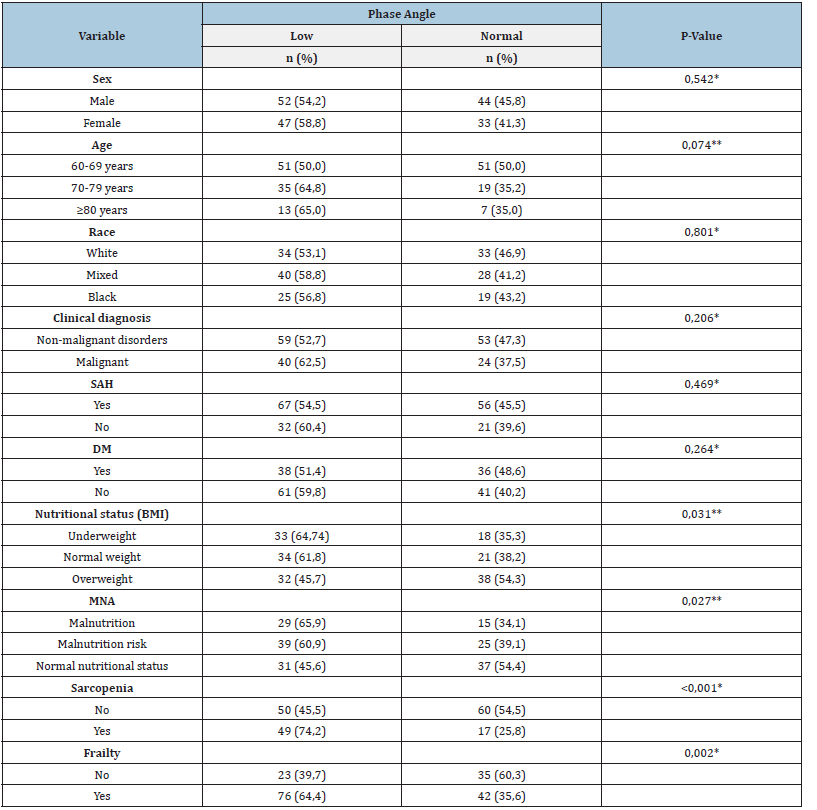
*Chi-Square test or**Linear Trend Chi Square. BMI: Body Mass Index; DM: Diabetes Mellitus; MNA: Mini Nutritional Assessment; SAH: Systemic Arterial Hypertension. Sarcopenia defined by the European Sarcopenia Consensus [3]; Frailty defined by Fried [5].
Table 3:Adjusted analysis of sarcopenia and frailty with low phase angle in hospitalized elderly patients (n=176).
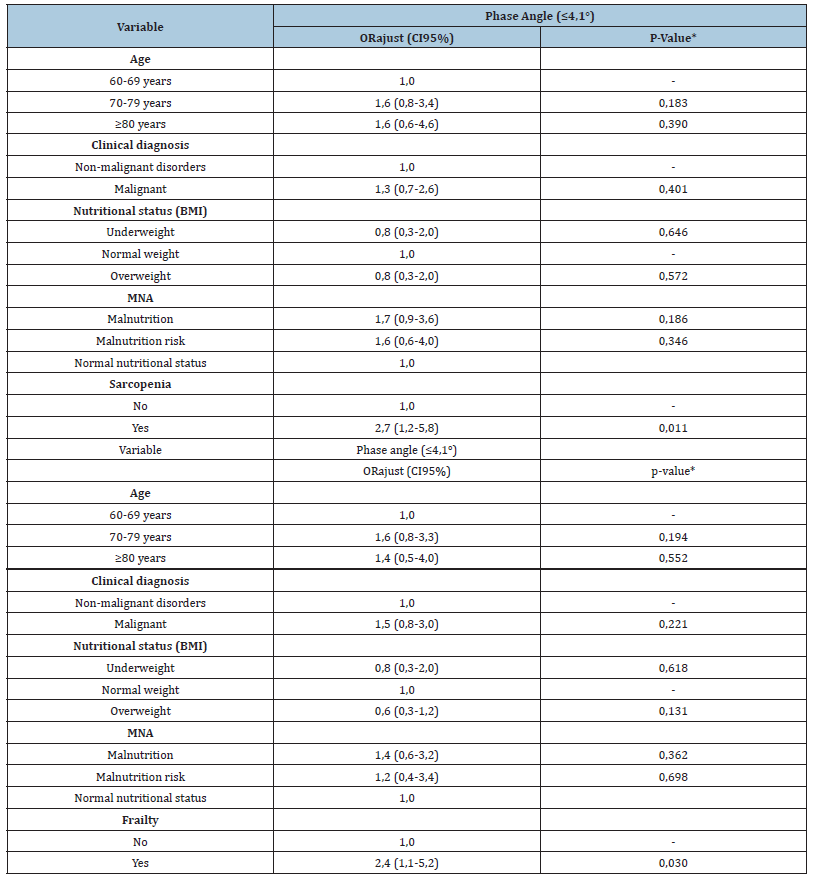
*Wald Test. BMI: Body Mass Index; CI 95%: 95% Confidence Interval; MNA: Mini Nutritional Assessment; ORajust: Adjusted Odds Ratio; Sarcopenia defined by the European Sarcopenia Consensus [3]; Frailty defined by Fried [5].
Table 4:Comparative analysis of phase angle values according to the presence of sarcopenia, frailty and changes in its components in elderly hospitalized patients (n=176).
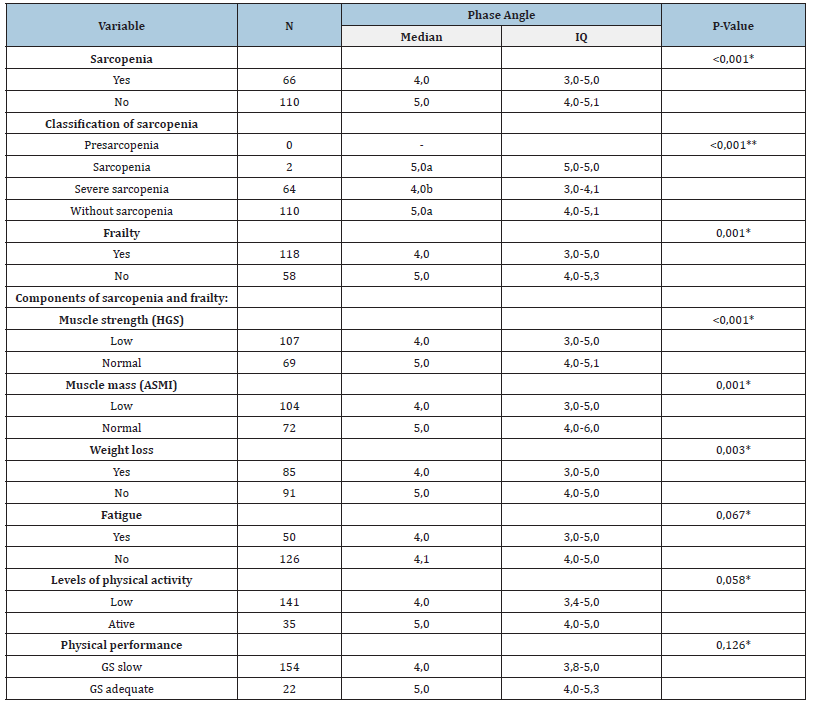
*Mann Whitney U Test ou **Kruskal Wallis. a,b Different letters mean statistical difference using the a posteriori Mann Whitney U test. ASMI: Appendicular Skeletal Muscle Mass; GS: Gait Speed; HGS: Hand Grip Strength; IQ: Interquartile; Sarcopenia defined by the European Sarcopenia Consensus³; Frailty defined by Fried [5].
Discussion
Sarcopenia and frailty are geriatric syndromes associated with a reduced quality of life and unfavorable health outcomes. Hospitalized individuals, especially those with limited mobility or in critical conditions, are particularly susceptible to nutritional challenges, including the gradual loss of muscle mass and functional capacity. In this context, it is essential to investigate these conditions using assessment strategies suitable for specific situations [1,29]. Our study highlighted substantial prevalence rates of underweight as indicated by BMI (29%) and the risk of malnutrition or undernutrition as assessed by the MAN criteria (61.4%). These findings emphasize the nutritional vulnerability faced by hospitalized individuals in the aging population. Comparable results were reported by Ortega et al [30], who examined 157 hospitalized older patients and found a prevalence of 27.8% for underweight and 53.5% at risk of malnutrition. Our finding revealed a substantial proportion of individuals with a low PhA (56.3%). Our results are consistent with the findings of Kwon et al [31]. They 31 examined 279 patients in a long-term care facility associated with Myongji Hospital in South Korea and reported a 50.1% prevalence of reduced PhA, with median PhA values of 3.65°. Interestingly, despite differences in ethnic backgrounds, the prevalence of reduced PhA appeared to be comparable. The notable prevalence of individuals with reduced PhA in our population can be explained by the micro and macroscopic alterations that take place in cell membrane structure, cellular function and mass during the aging process. These changes involve the accumulation of fat within skeletal muscle cells and the transition of muscle fibers, which ultimately lead to compromised contractility and a reduction in muscle strength [29]. Furthermore, common conditions often associated with the geriatric population, such as sarcopenia, cachexia, malnutrition and oxidative stress, contribute to an increase in extracellular fluids. This, in turn, results in membrane frailty, causing cellular damage and consequently leading to a decrease in PhA [32,33]. The high prevalence of both sarcopenia (37.7%) and frailty (67%) uncovered in our study highlights the susceptibility of individuals in a hospitalization context to geriatric syndromes. In a meta-analysis, Petermann & Rocha et al. [34] reported a global prevalence of sarcopenia ranging from 10 to 27% among the geriatric population. Meanwhile, another metaanalysis focused on hospitalized older individuals and found a 23% frequency of sarcopenia [35]. These variations in prevalence can be attributed to differences in diagnostic criteria, varying assessment cutoffs and the diverse characteristics of the studied populations.
In relation to frailty, certain studies [36,37] have documented prevalence rates of 39.3% and 47%, respectively, among hospitalized older individuals. These findings, coupled with the nutritional data, underscore the critical significance of diagnosing and closely monitoring both nutritional status and geriatric syndromes within this demographic. Early detection not only helps prevent associated complications but also facilitates timely intervention measures. The link between reduced PhA and malnutrition, assessed using both BMI and the MAN criteria, was similarly observed by Kilic et al. [7] in their study encompassing both outpatients and inpatients older individuals. The authors [7] likewise observed that reduced PhA was associated with other nutritional indicators, including calf circumference, highlighting the PhA value as a nutritional screening tool. Nevertheless, in our study, this association did not remain significant after accounting for confounding factors, indicating that it may be influenced by other reasons. In our study, we observed an independent association between reduced PhA and the diagnosis of sarcopenia. Individuals with sarcopenia had a 2.7 times higher likelihood of having a low PhA compared to those without sarcopenia. A similar finding was reported by Kilic et al. [7], who conducted a multivariate logistic regression analysis and found that sarcopenic individuals had a fourfold greater chance of having a low PhA [OR: 4.04 (95% CI: 1.55-10.52), p=0.04]. Additionally, Akamatsu et al. [32] investigated the potential of PhA in detecting sarcopenia and also observed lower PhA values in individuals with sarcopenia.
Our results further demonstrated lower PhA values in individuals with severe sarcopenia, suggesting that PhA may serve as an indicator of the severe sarcopenia. This could be due to more pronounced structural impairments in muscle tissue [29], resulting in decreased membrane function and increased permeability [17]. Similar findings have been reported by other researchers [7,18,38] in their studies involving hospitalized older patients. Furthermore, PhA has been associated with significant consequences in the geriatric population, including frailty, a heightened risk of falls, disability and mortality [11,31]. In line with our findings in relation to sarcopenia, our study also observed an independent association between low PhA and frailty [Adj OR: 2.4 (95% CI 1.1-5.2)]. These results are consistent with the findings of Wilhelm-Leen et al. [39], who reported that low PhA was independently associated with frailty and mortality in older individuals. However, divergent results were presented by Delgado et al. [40], who found that PhA was lower in frail individuals compared to non-frail individuals (5.4°vs6.1°, p=0.04), although this effect was not significant in adjusted analysis. It’s worth noting that the relationship between frailty and PhA has been relatively underexplored and additional research is needed to establish more consistent findings. PhA has garnered attention from several researchers due to its association with the aging process, which involves a link to reduced cellular function and membrane permeability [9,10,17]. In this context, PhA can prove to be a valuable tool for screening geriatric syndromes, particularly in situations where the necessary parameters for diagnosing these conditions cannot be obtained. It’s important to note that many researchers exclude individuals who are unable to undergo assessments for sarcopenia and frailty diagnosis, which could potentially lead to an underestimation of the prevalence of these geriatric syndromes. The phase angle cut off values to indicate sarcopenia in the studies, however, ranged considerably from 4° to 5° and it is not clear whether these differences were due to the population studied or due to the BIA device [41,42].
Acknowledging the limitations of this study is essential for a comprehensive interpretation of the results. It’s important to note that the study includes a specific population with unique characteristics and regional considerations. Furthermore, being a hospital-based study, the generalizability of the findings to geriatric individuals with different characteristics may be limited. Additionally, the study design is cross-sectional, which means that it cannot establish temporal relationships and has limited predictive capacity. However, it’s worth emphasizing that while the association between PhA and sarcopenia has been extensively explored, there have been relatively few studies investigating PhA as a marker for frailty.
Conclusion
PhA emerges as a promising marker for the identification of sarcopenia and frailty in hospitalized older individuals. Moreover, lower PhA values may serve as an indicator of the severity of sarcopenia. However, it is imperative to underscore the need for additional research to comprehensively assess the utility of PhA as a timely biomarker for geriatric syndromes. The validation of straightforward and adaptable tools across diverse clinical scenarios can significantly enhance the screening and early detection of both sarcopenia and frailty in this population.
References
- Divo MJ, Martinez CH, Mannino DM (2014) Ageing and the epidemiology of multimorbidity. European Respiratory Journal 44(4): 1055-1068.
- Manfredi G, Midão L, Paúl C, Cena C, Duarte M, et al. (2019) Prevalence of frailty status among the European elderly population: Findings from the survey of health, aging and retirement in Europe. Geriatrics & Gerontology International 19(8): 723-729.
- Cruz Jentoft AJ, Bahat G, Bauer J (2019) Sarcopenia: Revised European consensus on definition and diagnosis. Age and Ageing 48(1): 16-31.
- Kosoku A, Uchida J, Nishide S (2020) Association of sarcopenia with phase angle and body mass index in kidney transplant recipients. Scientific Reports 10(1): 266.
- Fried LP, Tangen CM, Walston J (2001) Frailty in older adults: Evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 56(3): M146-M156.
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K, et al. (2013) Frailty in elderly people. The Lancet 381(9868): 752-762.
- Kilic MK, Kizilarslanoglu MC, Arik G, Bolayir B, Kara O, et al. (2017) Association of bioelectrical impedance analysis-derived phase angle and sarcopenia in older adults. Nutrition in Clinical Practice 32(1): 103-109.
- Santiago LB, Roriz AKC, Oliveira CCD, Oliveira TMD, Conceição Machado MEP, et al. (2022) Phase angle as a screening method for sarcopenia in community-dwelling older adults. Journal of Nutrition 35(1).
- Rosas Carrasco O, Ruiz Valenzuela RE, López Teros MT (2021) Phase angle cut-off points and their association with sarcopenia and frailty in adults of 50-64 years old and older adults in Mexico City. Frontiersin Medicine 8: 617126.
- Mullie L, Obrand A, Bendayan M, Trnkus A, Ouimet MC, et al. (2018) Phase angle as a biomarker for frailty and postoperative mortality: The BICS study. Journal of the American Heart Association 7(17): e008721.
- Vincenzo Di O, Marra M, Gregorio Di A, Pasanisi F, Scalfi L, et al. (2021) Bioelectrical Impedance Analysis (BIA)-derived phase angle in sarcopenia: A systematic review. Clinical Nutrition 40(5): 3052-3061.
- Dos Reis AS, Santos HO, Limirio LS, Oliveira EP DE (2019) Phase angle is associated with handgrip strength but not with sarcopenia in kidney transplantation patients. Journal of Renal Nutrition 29(3): 196-204.
- Pessoa DF, Branco FM de, Dos Reis AS, Limirio LS, Borges LDP, et al. (2020) Association of phase angle with sarcopenia and its components in physically active older women. Aging Clinical and Experimental Research 32(8): 1469-1475.
- Hirose S, Nakajima T, Nozawa N, Katayanagi S, Ishizaka H, et al. (2020) Phase angle as an indicator of sarcopenia, malnutrition and cachexia in inpatients with cardiovascular diseases. Journal of Clinical Medicine 9(8): 2554.
- Norman K, Stobäus N, Pirlich M, Bosy Westphal A (2012) Bioelectrical phase angle and impedance vector analysis-clinical relevance and applicability of impedance parameters. Clinical Nutrition 31(6): 854-861.
- Lukaski HC, Kyle UG, Kondrup J (2017) Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Current Opinion in Clinical Nutrition and Metabolic Care 20(5): 330-339.
- Uemura K, Doi T, Tsutsumimoto K, Nakakubo S, Kim MJ, et al. (2020) Predictivity of bioimpedance phase angle for incident disability in older adults. Journal of Cachexia, Sarcopenia and Muscle 11(1): 46-54.
- Santana NDM, Pinho CPS, Da Silva CP, Dos Santos NF, Mendes RML, et al. (2018) Phase angle as a sarcopenia marker in hospitalized elderly patients. Nutrition in Clinical Practice 33(2): 232-237.
- Sánchez Iglesias A, Fernández Lucas M, Teruel JL (2012) The electrical basis of bioimpedance. Nefrología 32(2): 133-135.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, et al. (2004) Bioelectrical impedance analysis-part I: review of principles and methods. Clinical Nutrition 23(5): 1226-1243.
- Fess EE (1982) The effect of Jamar dynamometer handle position and test protcol on normal grip strength. J Hand Surg 7: 308-309.
- Sergi G, De Rui M, Veronese N, Bolzetta F, Berton L, et al. (2015) Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults. Clinical Nutrition 34(4): 667-673.
- Barbosa Silva TG, Bielemann RM, Gonzalez MC, Menezes AMB (2016) Prevalence of sarcopenia among community‐dwelling elderly of a medium‐sized South American city: Results of the COMO VAI? study. Journal of Cachexia, Sarcopenia and Muscle 7(2): 136-143.
- Batistoni SST, Neri AL, Cupertino APFB (2007) Validity of the center for epidemiological studies depression scale among Brazilian elderly. Revista de Saude Publica 41(4): 598-605.
- Matsudo S, Araújo T, Marsudo V, Andrade Andrade DE, Braggion G, et al. (2001) International physical activity questionnaire (IPAQ): A study of validity and reproducibility in Brazil. Rev Bras Act Fis Health, pp. 5-18.
- Bosch X, Monclús E, Escoda O, Guerra García M, Moreno P, et al, (2017) Unintentional weight loss: Clinical characteristics and outcomes in a prospective cohort of 2677 patients. PLoS One 12(4): e0175125.
- Lipschitz DA (1994) Screening for nutritional status in the elderly. Primary Care: Clinics in Office Practice 21(1): 55-67.
- Guigoz Y, Vellas B, Garry PJ (1996) Assessing the nutritional status of the elderly: The mini nutritional assessment as part of the geriatric evaluation. Nutrition Reviews 54(1 Pt 2): S59-65.
- Geng J, Wei Y, Xue Q, Deng L, Wang J, et al. (2022) Phase angle is a useful bioelectrical marker for skeletal muscle quantity and quality in hospitalized elderly patients. Medicine 101(45): e31646.
- Ortega SB, Redondo delRío P, CarreñoEnciso L, Cruz Marcos S De La, Massia MN, et al. (2023) Phase angle as a prognostic indicator of survival in institutionalized psychogeriatric patients. Nutrients 15(9): 2139.
- Kwon YE, Lee JS, Kim JY, Baeg SI, Choi HM, et al. (2023) Impact of sarcopenia and phase angle on mortality of the very elderly. Journal of Cachexia Sarcopenia and Muscle 14(1): 279-287.
- Akamatsu Y, Kusakabe T, Arai H, Yamamoto Y, Nakao K, et al. (2022) Phase angle from bioelectrical impedance analysis is a useful indicator of muscle quality. Journal of Cachexia, Sarcopenia and Muscle 13(1): 180-189.
- Bellido D, García García C, Talluri A, Lukaski HC, García Almeida JM, et al. (2023) Future lines of research on phase angle: Strengths and limitations. Reviews in Endocrine and Metabolic Disorders 24(3): 563-583.
- Petermann Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, et al. (2022) Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta‐analysis. Journal of Cachexia, Sarcopenia and Muscle 13(1): 86-99.
- Papadopoulou SK, Tsintavis P, Potsaki G, Papandreou D (2020) Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. a systematic review and meta-analysis. The Journal of Nutrition, Health & Aging, 24(1): 83-90.
- Veronese N, Custodero C, Cella A, Demurtas J, Zora S, et al. (2021) Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Research Reviews 72: 101498.
- Ligthart Melis GC, Luiking YC, Kakourou A, Cederholm T, Maier AB, et al. (2020) Frailty, sarcopenia and malnutrition frequently (co-) occur in hospitalized older adults: A systematic review and meta-analysis. Journal of the American Medical Directors Association 21(9): 1216-1228.
- Güner M, Ceylan S, Okyar BA, Kahyaoglu Z, Çoteli K, et al. (2023) Phase angle is associated with frailty in community-dwelling older adults. Nutrition 116: 112157.
- Wilhelm Leen ER, Hall YN, Horwitz RI, Chertow GM (2014) Phase angle, frailty and mortality in older adults. Journal of General Internal Medicine 29: 147-154.
- Delgado C, Doyle JW, Johansen KL (2013) Association of frailty with body composition among patients on hemodialysis. Journal of Renal Nutrition 23(5): 356-362.
- Norman K, Herpich C, Müller Werdan U (2023) Role of phase angle in older adults with focus on the geriatric syndromes sarcopenia and frailty. Rev Endocr Metab Disord 24(3): 429-437.
- Jian Zhang (2024) The diagnostic accuracy and cutoff value of phase angle for screening sarcopenia: A systematic review and meta-analysis. J Amer Med Dir Assoc 25(11): 105283.
© 2025 Cláudia Porto Sabino Pinho. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






