- Submissions

Full Text
Gerontology & Geriatrics Studies
Cerebral Angiogenesis and Neurogenesis in The Treatment of Ischemic Stroke in Elderly and Geriatric Patients
Ivan V. Maksimovich*
Clinic of Cardiovascular Diseases named after Most Holy John Tobolsky, Moscow, Russia
*Corresponding author:Ivan V. Maksimovich, Clinic of Cardiovascular Diseases named after Most Holy John Tobolsky, Moscow, Russia
Submission: September 23, 2024; Published: October 01, 2024

ISSN 2578-0093Volume9 Issue2
Abstract
Aim: The research is devoted to the clinical application of transcatheter intracerebral Laser Photo Bio Modulation Therapy (PBMT) for angiogenesis stimulation, cerebral collateral blood supply restoration, neurogenesis stimulation, cerebral tissues regeneration in ischemic stroke treatment.
Methods: 953 patients aged 30-80 (average age 74.6) with ischemic stroke were examined. There were 696 men (73.03%) 257 women (26.97%).
Test group: 610 (64.01%) patients underwent transcatheter intracerebral laser PBMT, of whom 354 (58.04%) had small focal stroke, 189 (30.98%) had medium focal stroke and 67 (10.98%) had macrofocal stroke.
Control group: 343 (35.99%) patients underwent conservative treatment, of whom 256 (74.64%) had small focal stroke, 43 (12.54%) had medium focal stroke and 44 (12.82%) had macrofocal stroke.
Results
Test group: Angiogenesis stimulation, cerebral collateral blood supply restoration, neurogenesis stimulation and cerebral regenerative processes were achieved in all 610 cases regardless of the patients’ age. Good clinical result was obtained in 313 (88.42%) patients with small focal strokes, in 114 (60.32%) patients with medium focal strokes, in 14 (20.90%) patients with macrofocal strokes. Satisfactory clinical result was obtained in 36 (10.17%) patients with small focal strokes, in 48 (25.39%) patients with medium focal strokes, in 27 (40.30%) patients with macrofocal strokes.Control group: Good clinical result was obtained in 51 (19.92%) patients with small focal strokes; patients with medium focal and macrofocal strokes showed no good result. Satisfactory clinical results were obtained in 63 patients (24.61%) with small focal strokes and in 8 patients (18.60%) with medium focal strokes. No satisfactory results were obtained in patients with macrofocal strokes.
Conclusion: Angiogenesis and neurogenesis are important directions in ischemic stroke treatment. Transcatheter intracerebral laser PBMT stimulates these processes and is an effective, pathogenetically substantiated method for ischemic stroke treatment. The method normalizes cerebral blood supply, causes tissue regeneration, which leads to the restoration of daily life, cognitive and mental functions in young as well as in elderly and geriatric patients.
Keywords:Ischemic stroke; Therapeutic targets; Transcatheter intracerebral laser photobiomodulation therapy; PBMT; Angiogenesis; Neurogenesis; Regeneration
Introduction
In recent years, ischemic stroke has become one of the most common causes of patients’ severe disability and death. In the United States alone 159,050 people died from stroke in 2020 [1]. Ischemic stroke is actively spreading: 7.59 million cases were registered in all countries in 2020 [2]. The brain is the most actively blood supplied organ in the human body, it has complex angioarchitecture and microcirculation [3,4]. The large arteries carry out a transporting function and only deliver blood to the cerebral peripheral bed. It is the intracerebral capillary bed that plays the main role in cerebral blood supply, oxygenation and in ensuring neuronal metabolism [3,4]. One cubic centimeter of cerebral tissue contains about 3-4 thousand capillaries [3]. The brain is extremely sensitive to decreased blood supply and subsequent hypoxia, which very quickly leads to ischemia, death of cerebral tissue and stroke development [3-7]. The more extensive the ischemic zone is, the more it spreads to various cerebral regions, the more severe the stroke and its consequences are [3, 5, 6]. The main cause of cerebral ischemia is atherosclerosis, which leads to stenosis, subsequent thrombosis and occlusion of extracranial, intracerebral arteries, arterioles and capillaries [4,6]. The extracranial type of atherosclerotic lesion occurs in 9.33% of cases, the mixed type in 46.19% of cases, the intracerebral type in 44.53% of cases [6]. The development of cerebral ischemia and ischemic stroke cause both destructive and restorative processes in the brain [3,6,8,9]. Under natural conditions, the organism strives to reduce the damage caused and responds to ischemia development with a complex of complicated reactions aimed at revascularization and restoration of cerebral tissue. A decrease in blood flow in intracerebral arteries and capillaries stimulates angiogenesis, which leads to the development of the collateral bed. The collateral bed delivers blood to the ischemic areas of the brain from other, unaffected arterial basins [3,4,6,8,9]. With aging, cerebral blood supply decreases, which stimulates angiogenesis, leading to the development of the collateral bed. As a result, older patients have milder ischemic strokes than younger ones, in whom collateral blood supply has not yet developed [3,4,6-8].
Simultaneously, angiogenesis and collaterals development are neuroprotectors that stimulate the processes of neurogenesis and regeneration of cerebral tissues [3-6,8-11]. Unfortunately, these natural physiological processes occur quite slowly and after an ischemic stroke, they do not lead to rapid, clinically significant recovery [6]. Many authors note the extremely important significance of stimulating cerebral angiogenesis and neurogenesis in the treatment of cerebrovascular insufficiency and ischemic stroke [2,4,6-11]. Conservative treatment methods common in modern practice do not have clear directions of affecting cerebral tissues. The effectiveness of existing drugs is quite limited [4,6,8,9]. As a result, conservative treatment is usually effective for small focal strokes with minor areas of neurosecretion and neurodegeneration [4,12]. For more extensive ischemic strokes, as well as those in elderly patients, conservative treatment becomes less effective [6,9,11-13]. A particular group in the treatment of ischemic stroke is stem cell therapy methods. However, this promising area is still under development [9,10].
Conducting interventional and reconstructive surgeries is aimed at improving the main blood supply and is usually carried out on large arterial trunks. These methods have proven themselves effective in operations on brachiocephalic arteries in younger patients [13-17]. In case of intracerebral atherosclerotic lesions, these interventions are difficult to carry out due to the small diameter and intracranial location of the vessels [6,14,15]. Systemic and local thrombolysis, transcatheter mechanical thrombectomy and thromboextraction are also aimed at restoring the main cerebral blood supply. These methods are advisable to be used only in the acute stage of ischemic stroke [18,19]. When using these methods, certain difficulties arise in determining the neuroprotective effect [20]. This situation in the treatment of cerebrovascular lesions and ischemic stroke requires the development of new, effective methods aimed at both dealing with various processes that occur during the development of hypoxia, ischemia and neurosecretion and at stimulating recovery processes. These methods should include a comprehensive impact on angiogenesis, neuroprotection and neurogenesis [4,6-9]. One of the promising areas in the treatment of cerebrovascular lesions and ischemic stroke is the use of laser energy [4,6,7,21-30]. The use of laser energy for medical purposes began almost immediately after the discovery of the laser effect, in the 1960s. In 1967, E. Mester et al. were the first to use a ruby laser with low output power to restore hair covering [31]. In 1968, N. S. Makeeva and V. V. Schur successfully used a helium-neon laser to heal superficial skin wounds [32].
Currently, this direction in laser medicine, using a laser with low
output power, is called laser Photobiomodulation Therapy (PBMT)
[4,7,21-24]. In the treatment of ischemic, neurodegenerative and
traumatic cerebral lesions, PBMT uses a laser with low output
power of the red or near-infrared spectral range (wavelength
600-1100nm). With PBMT, laser exposure has a precise direction
and has a multicomponent, complex effect on cerebral tissues
[22,23,24]. Laser energy acts at the molecular, cellular and tissue
levels. This effect stimulates the formation of active oxygen forms,
increases mitochondrial oxygen consumption, restores Adenosine
Triphosphate (ATP) metabolism in mitochondria, stimulates and
restores metabolic processes in neurons, stimulates angiogenesis,
causes the opening of collateral vessels, improves cerebral blood
flow, eliminates vascular dysfunction, increases tissue oxygenation,
stimulates lymphatic drainage, improves blood flow, reduces
apoptosis, stimulates neurogenesis and synaptogenesis, causes
cerebral tissue regeneration and the development of intercellular
connections [4,6,7,21-29]. The energy of the laser with low
output of the red spectral range, when directly applied to the brain,
deeply penetrates the cerebral tissue. At a wavelength of 600 to
700nm, the penetration depth is 20-40mm [33]. At a wavelength of
808nm, it is 40-50mm [34]. For various brain lesions, laser PBMT
includes transcranial [7,22,23] intranasal (often in combination
with transcranial) [ 24,25,35] intravascular (intravenous) [36] и
and transcatheter intracerebral treatment methods [4,6,21,30,33]:
A. Transcranial is a non-invasive, fairly simple method. Laser
energy is supplied to the scalp; special helmets with several
LED emitters are often used to increase the area of exposure
[7,22,23,26,27]. To reach the brain, laser energy passes through
the tissues of the scalp, skull bones and meninges. These
structures have a high degree of absorption and a fairly low
degree of light transmission, which reduces the level of laser
energy reaching the brain. As a result, a limited amount of
energy reaches the cerebral tissues. In this regard, to obtain
a stable clinical effect, it is necessary to conduct a sufficiently
large number of sessions [22,23,29]. The treatment results do
not depend on the patients’ age.
B. Intranasal (sometimes transpenoidal) is also a fairly
simple, minimally invasive method. Laser energy is supplied
to the brain through the nasal cavities [24]. Laser energy
passes through the mucous membranes of the nose and the
thinner bones of the skull. These tissues have a lower degree
of absorption and a higher degree of transmission of laser
energy. With this method of supply, more energy reaches the
brain. But this method also requires a sufficiently large number
of sessions. For a total increase in laser energy penetrating
into the brain tissue and enhancing the therapeutic effect, the
intranasal method is often combined with the transcranial
method [25, 35]. The treatment results do not depend on the
patients’ age.
C. Intravascular (intravenous) is a fairly simple, minimally
invasive method. During its implementation, through a
peripheral intravenous catheter is inserted a fiber optic lightguiding
instrument, through which the blood and vascular wall
are exposed to the laser. There is no direct effect of laser energy
on brain tissue. According to the authors, the method provides
a pronounced clinical effect in patients who have suffered their
first limited ischemic stroke. To obtain a stable positive clinical
effect, at least 10 sessions are necessary [36]. The treatment
results do not depend on the patients’ age.
D. Transcatheter intracerebral is also a minimally invasive,
interventional, but more complex method. The intervention
is carried out in catheterization laboratories. Under
fluoroscopic control, a peripheral artery is catheterized. Using
interventional technology, a flexible small-diameter laser fiberoptic
light-guiding instrument connected to the laser unit is
passed through the peripheral and then carotid arteries to
the affected intracerebral arterial branch. Laser exposure is
performed through this fiber-optic instrument. Due to the
greater amount of laser energy, one intervention is sufficient to
obtain a pronounced positive clinical effect [4,6,30,33,37]. The
treatment results do not depend on the patients’ age.
The present study is devoted to the clinical application of transcatheter intracerebral laser Photobiomodulation Therapy (PBMT) for stimulation of angiogenesis, restoration of cerebral collateral arterial and capillary blood supply, stimulation of neurogenesis and regeneration of cerebral tissues in the treatment of ischemic stroke in young patients and in elderly and geriatric patients.
Materials and Methods
Diagnostics and intracerebral transcatheter laser interventions, as well as conservative treatment in this research, were performed with the approval of the ethics committee (Protocol No. 2 of 01- 04-1996, Protocol No. 4 of 01-12-2002, Protocol No. 12 of 03-26- 2015, Protocol No. 12 of 04-15-2021) and the consent of patients and their relatives.
Patient selection criteria:
a) consent of patients and their relatives to the necessary
examination and treatment.
b) patients’ somatic condition allowing the necessary
examination and treatment.
c) patients’ cerebral ischemic stroke history in the period
from 6 months to 6 years.
d) cerebral structural changes, signs of daily life
deterioration, cognitive impairment and dementia in patients.
Patient examinations
A. Cerebral structural changes were determined using CT
and MRI according to generally accepted schemes.
B. Daily life activities were assessed using the Bartels
Functional Evaluation (IB) Index [38].
C. Cognitive functions were assessed using the Mini-Mental
State Examination (MMSE) [39].
D. The severity of dementia was assessed using the Clinical
Dementia Rating scale (CDR) [40].
E. Laboratory examination: coagulological, biochemical and
general clinical tests.
F. Cerebral blood flow was assessed using Scintigraphy (SG)
with TC 99M Pertechnetate 555.
G. Impaired pulse blood filling in the cerebral hemispheres
was assessed using Rheoencephalography (REG).
H. The condition of the cerebral vascular and microcirculatory
bed was assessed using cerebral Multi-Gated Angiography
(MUGA) in the direct and lateral projections [6,30]. Capillary
blood flow was determined using the computer program “Angio
Vision” [4,33,41-44]. In some cases, Multiperil Computed
Tomography Angiography (MSCTA) or Magnetic Resonance
Angiography (MRA) were carried out in the remote period.
Analysis of Patients
953 patients aged 30-80 (mean age 74.6) who had had an ischemic stroke in the period from 6 months to 6 years were examined: 696 (73.03%) were men and 257 (26.97%) were women.
Test group-
610 (64.01%) patients underwent transcatheter intracerebral
laser PBMT of whom:
a) 354 (58.04%) patients with small focal stroke.
b) 189 (30.98%) patients with medium focal stroke.
c) 67 (10.98%) patients with macrofocal stroke.
Control group-
343 (35.99%) patients underwent conservative treatment, of
whom:
a) 256 (74.64%) patients with small focal stroke.
b) 43 (12.54%) patients with medium focal stroke.
c) 44 (12.82%) patients with macrofocal stroke.
All patients in the test and control groups had similar age, somatic conditions, stroke severity, time after stroke and postischemic cyst sizes.
The results of the initial examination of patients in the study and control groups are presented in Table 1.
Table 1:Patient examination data
Test Group
Patients in the test group underwent Transcatheter Intracerebral Laser Photobiomodulation Therapy (PBMT). Under local anesthesia, using the Selinger method, the common femoral artery is catheterized. A guiding microcatheter is coaxially inserted and led across the vascular bed through the carotid artery to the affected intracerebral branches. A flexible fiber-optic light-guiding instrument, with a diameter of 25 to 100 micrometers, connected to a helium-neon laser (wavelength 632.8nm), is inserted through this catheter. Thanks to this light-guided instrument, intracerebral laser exposure is made possible (the laser exposure characteristics: fiber output power of 24-45mW, beam spot diameter in the vessel of 1-2mm, average dose during treatment of 29-106J, treatment session duration of 1200-2400 seconds) [30,37,42-44]. This method allows for targeted, direct exposure to the endothelium, vascular wall, blood and surrounding cerebral tissues. The insignificant thickness of the fiber-optic instruments allows for working with small-diameter intracerebral arterial branches, which significantly distinguishes this method from other interventional treatment methods. Since atherosclerosis is a systemic disease, it affects intracerebral arteries, arterioles and capillaries of both hemispheres. The severity of the lesion may vary; in one hemisphere, atherosclerotic changes may be more pronounced than in the other. But in the hemisphere with less pronounced atherosclerosis, vascular changes that reduce intracerebral blood flow still exist and they contribute to hypoxia and ischemia [3-5,8,12,13]. For this reason, to improve the blood supply to the entire brain, transcatheter intracerebral PBMT was performed in all cases on both sides, in both the right and left hemispheres [30,41-44]. At the end of the transcatheter intracerebral intervention, a repeated cerebral MUGA is performed. The results of MUGA are used to determine the severity of intracerebral angiogenesis, the degree of collateral, capillary revascularization. After transcatheter, intracerebral laser PBMT, the patients underwent complex antiplatelet, anticoagulant, antioxidant, vasodilator and nootropic therapy. In accordance with the parameters of the blood coagulation system, the patients took Aspirin, Heparin and indirect anticoagulants. Intravenously, they took 10-15 infusions of Pentoxifylline 100mg, Complamin 150mg, Inosin 200mg, Nootropil (Piracetam) 1200mg (or Gliatilin 1000mg) followed by pills. In subsequent periods of treatment, courses of infusions and pills were repeated 2 times a year for 1-2 months.
Control Group
The patients of the control group underwent conservative treatment using similar regimens and doses of drugs used in the postoperative period by patients of the test group. In accordance with the parameters of the blood coagulation system, the patients took Aspirin, Heparin, indirect anticoagulants, 10-15 infusions of Pentoxifylline 100mg, Complamin 150mg, Inosin 200mg, Nootropil (Piracetam) 1200mg (or Gliatilin 1000mg) followed by pills. In the subsequent period, the patients also underwent repeated courses of infusions and pills 2 times a year for 1-2 months.
Evaluation of Results After the Treatment
The results obtained after the treatment were considered as:
a) Good clinical result-almost complete recovery of motor,
cognitive and mental functions (IB 95-100, MMSE 27-30, no
dementia).
b) Satisfactory clinical result-incomplete recovery of motor,
cognitive and mental functions (IB 85-90, MMSE 25-27, the
level of dementia has decreased).
c) Relatively satisfactory clinical result-partial recovery
of motor, cognitive and mental functions (IB below 80, MMSE
below 25, the level of dementia has decreased but it remains).
d) Relatively positive clinical result-no negative dynamics
with minor recovery of motor, cognitive and mental functions.
Results
Test group
Immediate results: According to cerebral angiography (MUGA), after transcatheter intracerebral laser PBMT, an immediate positive result, manifested by pronounced angiogenesis, collateral and capillary revascularization, was obtained in 608 (99.67%) patients (Figures 1B, 1C, 2B, 2C, 3B, 3C). In 2 (0.33%) patients, angiogenesis was manifested to a lesser extent. Complications associated with these interventions were not observed.
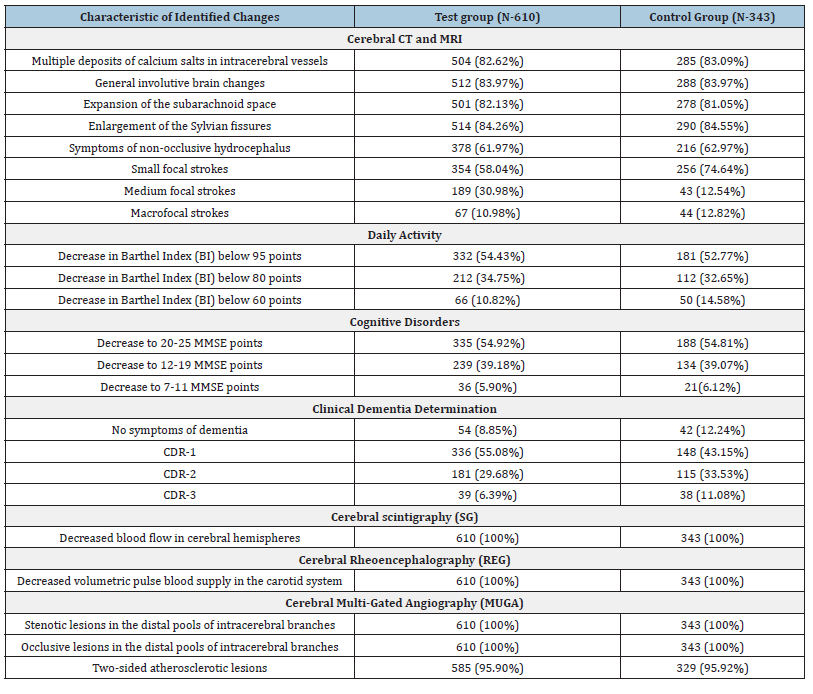
Figure 1:Patient H., female, 60 years old. Atherosclerosis of cerebral vessels, chronic cerebrovascular insufficiency
ischemic stroke in the basin of the middle cerebral artery to the right. Before and after transcatheter intracerebral
PBMT.
A. CT of the brain, before the intervention: 1. moderate heterogeneous postischemic cyst in the right middle
cerebral artery region. 2. moderate expansion of the subarachnoid space.
B. Right internal carotid artery angiogram, arterial phase, before the intracerebral laser PBMT: 3. occlusion of
distal branches of the right middle cerebral artery; 4. multiple stenosis of intracranial branches.
C. Right internal carotid artery angiogram, arterial phase, after the intracerebral laser PBMT: 5. Stimulation of
angiogenesis, collateral arterial and capillary revascularization of the right hemisphere.
D. CT of the brain, 12 months after the intracerebral laser PBMT: 6. reduction in the size of the post-ischemic
cyst with signs of cerebral tissue structure recovery; 7. subarachnoid space restoration.
E. CT of the brain, 6 years after the intracerebral laser PBMT: 8. the structure of the right hemisphere cerebral
tissue is restored, no signs of residual effects of the post-ischemic cyst.
F. MRA of the brain. 10 years after the intracerebral laser PBMT: the patency and lumen of the distal
branches of the right internal carotid artery are completely preserved, there is further progression of collateral
revascularization.
Early period (1-6 months) after transcatheter intracerebral laser PBMT: According to the results of SG and REG, improvement of cerebral blood flow and volumetric pulse blood filling in the hemispheres was observed in all 610 (100%) patients. According to the results of CT and MRI, a tendency to a decrease in general involutional changes in the brain, as well as narrowing of the subarachnoid space and a decrease in the volume of post-stroke cysts, were observed in all 610 (100%) patients (Figures 1A, 1D, 2A, 2D). According to the results of testing (IB, MMSE and CDR), pronounced positive clinical dynamics was observed in all 610 (100%) patients.
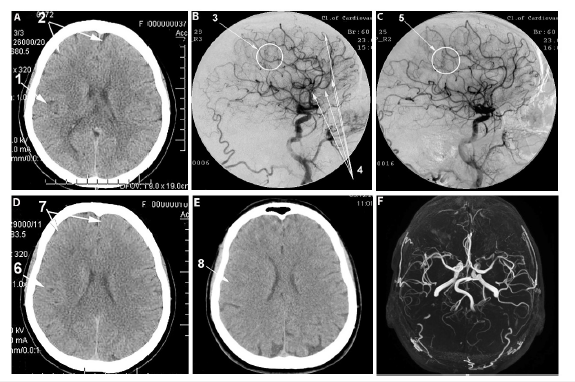
Figure 2:Patient A., female, 58 years old (BI-50, CDR-2). Atherosclerosis of cerebral vessels, chronic
cerebrovascular insufficiency, extensive ischemic stroke in the occipital parietal region of
the right hemisphere. Before and after transcatheter intracerebral PBMT.
A. MRI of the brain, before the intervention: 1. extensive postischemic cyst in the right occipital parietal region
of the right hemisphere.
B. Right internal carotid artery angiogram, arterial phase, before the intracerebral laser PBMT: 2. multiple
occlusions of the distal branches of the right medial cerebral artery.
C. Right internal carotid artery angiogram, arterial phase, after the intracerebral laser PBMT: 3. stimulation of
angiogenesis, marked collateral arterial and capillary revascularization of the right hemisphere.
D. MRI of the brain, 10 months after the intracerebral laser PBMT: 4. significant reduction in the size of the
post-ischemic cyst with signs of cerebral tissue structure recovery.
Remote period (1-10 years) after transcatheter
intracerebral laser PBMT: According to the results of repeated
SG and REG, 1-2 years after PBMT, the improvement in cerebral
blood flow and volumetric pulse filling obtained in the early period
was observed in all 610 (100%) patients and was maintained
throughout the entire observation period. According to the results
of repeated CT and MRI, 1-2 years after PBMT
a) Of 512 patients (Table 1) a decrease in general involutional
changes in the cerebral cortex was observed in 491 (95.90%)
cases (Figures 1A,1D,2A,2D).
b) Of 501 patients (Table 1), narrowing of the subarachnoid
space was observed in 479 (95.61%) cases.
c) Of 514 patients (Table 1) narrowing of the Sylvian fissures
was observed in 493 (95.91%) cases.
d) Of 378 patients (Table 1) a decrease in the symptoms of
non-occlusive hydrocephalus was observed in 242 (64.02%)
cases.
e) A decrease in the volume of post-ischemic cysts after
stroke was observed in all 610 patients. Of these, a decrease
of 5-15% was noted in 71 (11.64%) cases, 15-25% in 206
(33.77%) cases, more than 25% in 333 (54.59%) cases (Figures
1A, 1D, 1E, 2A, 2D, 3A, 3D).
In the subsequent period (over 2 years), positive dynamics were maintained throughout the entire observation period in all 610 (100%) patients (Figures 1A, 1E, 3A, 3D). According to the results of repeated testing (IB, MMSE and CDR) in the subsequent period of over 2 years, pronounced positive clinical dynamics was observed in all 610 (100%) patients. According to the results of repeated MUGA, MSCTA, MRA in the period of 2 - 10 years after the performed PBMT, 232 (38.03%) patients were examined, with angiogenesis, collateral and capillary revascularization persisting in 229 (98.71%) cases (Figures 1C, 1F).
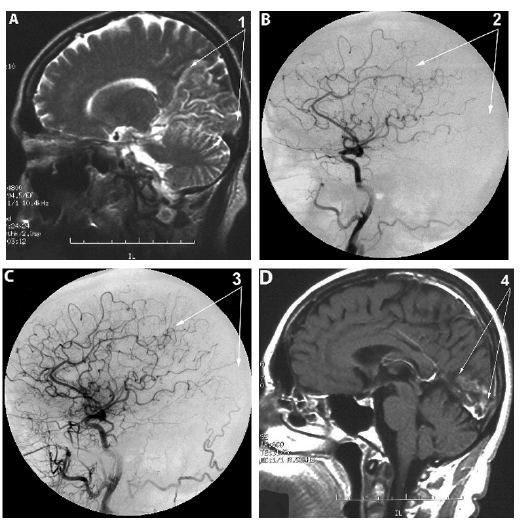
Figure 3:Patient J, male, 41 years old, (BI-40 CDR-3). Atherosclerosis of cerebral vessels, macrofocal ischemic
stroke of the right hemisphere before and after transcatheter intracerebral laser PBMT.
A. CT of the brain, before the intracerebral PBMT: 1. huge postischemic cyst in the right middle cerebral artery
region.
B. Right internal carotid artery angiogram, arterial phase, before the intracerebral laser PBMT: 2. Occlusion
of the right middle cerebral artery.
C. Right internal carotid artery angiogram, arterial phase, after the intracerebral PBMT: 3. Pronounced
collateral and capillary revascularization of the right hemisphere with good peripheral blood flow.
D. CT of the brain, 10 years after intracerebral PBMT: 4. Preservation and progression of pronounced
collateral revascularization of the right hemisphere with good peripheral blood flow (BI-100).
Control Group
Immediate results
After the course of conservative treatment, negative dynamics was not observed in any of 343 (100%) patients. Stabilization of the condition was observed in 78 (22.74%) cases. Positive dynamics, manifested by a moderate decrease in motor and mental disorders, was observed in 265 (77.26%) cases.
Early period (1-6 months) after the conservative treatment
According to the results of repeated SG and REG, unstable
improvement of cerebral blood flow and volumetric pulse blood
filling was observed:
a) in 204 (79.69%) cases out of 256 patients (Table 1) with
small focal strokes.
b) in 14 (32.56%) cases out of 43 patients (Table 1) with
medium focal strokes.
c) in 8 (18.18%) cases out of 44 patients (Table 1) with
macrofocal strokes.
According to the results of CT and MRI, there were no signs of a significant decrease in involutional cerebral changes, narrowing of the subarachnoid space, Sylvian fissures, a decrease in the phenomena of non-occlusive hydrocephalus or a decrease in the volume of post-stroke cysts in any of the 343 (100%) patients.
According to the results of repeated IB, MMSE and CDR testing,
partial improvement of motor and mental functions was observed:
a) in 238 (92.97%) cases out of 256 patients (Table 1) with
small focal strokes.
b) in 11 (25.58%) cases out of 43 patients (Table 1) with
medium focal strokes.
c) in 7 (15.91%) cases out of 44 patients (Table 1) with
macro focal strokes.
The remaining 87 (25.36%) patients showed stabilization of their condition.
Remote period (1-10 years) after the conservative treatment
According to the results of SG and REG performed 1-2 years
after conservative treatment
a) of 204 patients with small focal strokes who showed
positive dynamics in the early period, the indicators decreased
in 113 (55.39%) cases.
b) of 14 patients with medium focal strokes who showed
positive dynamics in the early period, the indicators decreased
in 7 (50.00%) cases.
c) of 8 patients with macrofocal strokes who showed
positive dynamics in the early period, the indicators decreased
in 3 (37.50%) cases.
d) The remaining 117 (34.11%) patients showed instability
of indicators.
In the subsequent period (over 2 years), all 343 patients showed a tendency towards a decrease in indicators.
According to the results of CT and MRI performed 1-2 years after the conservative treatment, significant signs of a decrease of involutional changes in brain tissue, narrowing of the subarachnoid space, narrowing of the Sylvian fissures, a decrease of non-occlusive hydrocephalus were not observed in any of the 343 patients. A decrease in the volume of post-ischemic cysts was not observed in any of the 343 patients. In the subsequent period (over 2 years), of all 343 patients in the control group, an increase in involutional changes, widening of the Sylvian fissures and symptoms of nonocclusive hydrocephalus were observed in 249 (72.59%) cases. According to the results of repeated IB, MMSE and CDR testing in the period of 1-2 years, the indicators of positive clinical dynamics with improvement of motor and mental functions changed.
The improvement was noted:
a) in 238 (92.97%) cases out of 256 patients (Table 1) with
small focal strokes.
b) in 12 (27.91%) cases out of 43 patients (Table 1) with
medium focal strokes.
c) in 7 (15.91%) cases out of 44 patients (Table 1) with
macrofocal strokes.
The remaining 86 (25.07%) patients showed stabilization of their condition.
According to the results of repeated testing (IB, MMSE and CDR) in the subsequent period of over 2 years, a decrease in the indicators was observed in all 343 patients (Table 2).
Table 2:Clinical Results in the remote period (1-10 years) after the treatment.
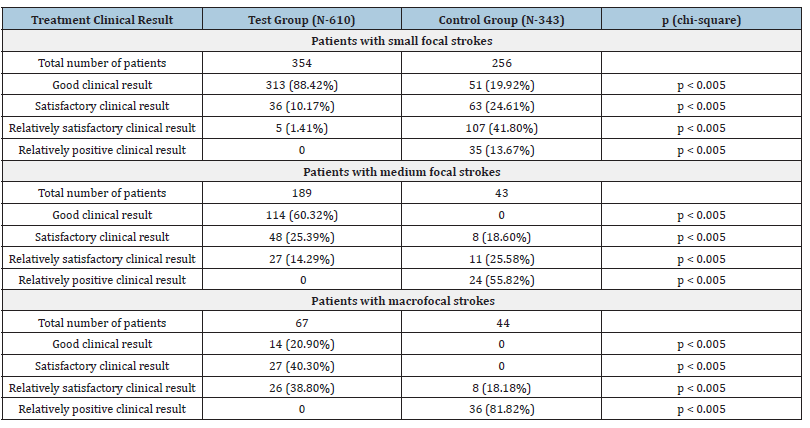
Due to well-known criteria and information on statistical data processing in medical research and practice, the differences between groups of patients are determined according to the analysis of the relevant 2 х 2 contingency tables using the Pearson chi-square test. The corresponding p-values are listed in the last column of the table. p = 0.05.
Discussion
Regardless of the patient’s age, treatment of ischemic stroke and its consequences should be physiologically justified, multicomponent, complex, directed at stimulation of angiogenesis and neurogenesis for revascularization and regeneration of cerebral tissues [4,6,8,9,37]. Common methods of conservative treatment of ischemic stroke consequences often do not have clear, specific directions of affecting cerebral tissues and at the same time, they are limited by the insufficient effectiveness of existing drugs [4,9]. Modern drugs used in clinical practice can improve blood supply, but do not stimulate angiogenesis and do not allow achieving pronounced, persistent cerebral revascularization. Modern drugs can affect metabolic and neuroprotective processes, but do not cause neurogenesis and regeneration of cerebral tissue. This often leads to the fact that against the background of the therapy, it is not always possible to achieve a pronounced therapeutic effect. In patients of the control group, repeated SG and REG performed at different stages of conservative treatment showed no pronounced, persistent improvement in cerebral blood supply and pulse blood filling. Unstable improvement in the indicators was observed mainly in patients with small-focal and medium focal strokes in the early period of treatment (1-6 months). In the late period (1-10 years), the obtained positive dynamics gradually decreased. Repeated CT and MRI carried out at different stages of treatment showed no signs of a decrease in involutional changes or the development of regenerative processes in cerebral tissue. Repeated IB, MMSE and CDR testing at different stages of treatment showed positive dynamics in daily life, cognitive functions and the level of dementia in a limited number of patients with small-focal and medium-focal strokes. As a result, in the control group, good and satisfactory clinical results were observed only in 114 (44.53%) patients with small-focal and in 8 (18.60%) patients with medium focal ischemic stroke. The obtained results are consistent with the data of other authors [2,9].
Brain tissue is an optically active medium [33]. In ischemic, neurodegenerative and traumatic cerebral lesions, lasers with low output power of the red or near-infrared spectral range have a complex mechanism of affecting cerebral tissues. As shown by numerous studies conducted in various countries, laser energy has a targeted effect on brain tissue. The effect occurs at the molecular, cellular and tissue levels, causing physiological angiogenesis and neurogenesis. As a result, regardless of patients’ age, vascular dysfunction is eliminated, collateral arterial and capillary revascularization develops, cerebral blood flow improves, tissue oxygenation increases, venous outflow is normalized, apoptosis decreases and regenerative processes in cerebral tissue develop [4,6,7,18,21-23,26,29,37]. Transcatheter intracerebral laser PBMT allows to accurately, listlessly deliver the required amount of laser energy to the site of cerebral damage, directly affecting the endothelium, vascular wall and surrounding cerebral tissues. In the test group of patients, repeated cerebral angiographic studies (MUGA, MSCTA and MRA), performed at different times after transcatheter intracerebral laser PBMT, showed persistent, pronounced collateral cerebral revascularization in 229 (98.71%) cases out of 232 re-examined patients.
Repeated cerebral SG, REG in the early (1-6 months) and late periods (1-10 years) showed that stimulation of angiogenesis with laser energy leads to a long-term, stable improvement in cerebral blood flow and volumetric pulse blood filling in all 610 (100%) cases. Repeated cerebral CT and MRI in the early period (1-6 months) already showed a decrease in involutional changes and a tendency to restoration of cerebral tissues in all 610 (100%) cases. In the late period (1-10 years) after transcatheter intracerebral laser PBMT, repeated cerebral CT and MRI clearly showed a decrease in involutional changes in the cerebral cortex, narrowing of the subarachnoid space, narrowing of the Sylvian fissures and a decrease in the phenomena of non-occlusive hydrocephalus and also a decrease in the size of the post-ischemic cyst with the restoration of normal cerebral tissue in all 610 (100%) cases. This dynamic indicates the development of cerebral regenerative processes. As a result, regardless of age, the patients of the test group showed restoration of their daily life (IB), cognitive functions (MMSE), as well as a decrease in dementia symptoms (CDR). In the remote period, regardless of age, the patients of the test group showed positive dynamics in 610 (100%) cases and good and satisfactory clinical results were obtained in 552 (90.49%) cases in patients with small focal strokes, medium focal strokes and macrofocal strokes (Table 2). showed positive dynamics in 610 (100%) cases and good and satisfactory clinical results were obtained in 552 (90.49%) cases in patients with small focal strokes, medium focal strokes and macrofocal strokes (Table 2). The obtained results confirm the data of other authors who showed positive results in the implementation of various PBMT methods for the treatment of the consequences of ischemic stroke [7,22-29].
Conclusion
Angiogenesis and neurogenesis are an important direction in the treatment of ischemic stroke. By stimulating angiogenesis and neurogenesis, transcatheter intracerebral laser PBMТ is a physiologically and pathogenetically substantiated, effective method for treating ischemic stroke and its consequences, regardless of the patients’ age. By stimulating angiogenesis, laser energy opens collateral arterial and capillary beds and, thereby, causes revascularization of the ischemic hemisphere. Simultaneous intracerebral PBMТ in the opposite hemisphere leads to normalization of blood supply to the entire brain.
Deeply affecting cerebral tissues, transcatheter intracerebral laser PBMТ, regardless of the patients’ age, stimulates neurogenesis. Laser energy improves cellular and tissue metabolism, restores ATP metabolism in neuronal mitochondria and produces active oxygen forms. Simultaneously, synaptogenesis is stimulated, and intercellular connections are restored. This multicomponent effect leads to the regeneration of cerebral tissues. Complex and low-traumatic effect of transcatheter intracerebral laser PBMТ in ischemic strokes of varying severity, regardless of the patients’ age, allows to achieve rapid and stable rehabilitation of patients. As a result, patients have their daily life and cognitive functions restored and symptoms of dementia reduced, which allows them to return to an independent, active, full life. The positive clinical effect obtained after transcatheter intracerebral laser PBMТ is observed for a long time, both in younger patients and in elderly and geriatric patients, which significantly distinguishes this method from other methods of treatment. Conservative treatment methods generally provide a positive clinical result in younger patients with mild forms of cerebral ischemic damage. The positive result is usually observed after small focal or medium focal strokes.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Ahmad FB, Anderson RA (2021) The leading causes of death in the us for 2020. JAMA 325(18): 1829-1830.
- Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, et al. (2023) Heart disease and stroke statistics-2023 update: A report from the American heart association. Circulation 147(8): e93-e621.
- Gjulev NM, Pustozertsev VG, Gjulev SN (2002) Cerebrovascular diseases. Binom, Moscow, Russia.
- Maksimovich IV (2019) Transcatheter intracerebral photo biomodulation in ischemic brain disorders: clinical studies (Part 2). Photobiomodulation in the Brain. Edited by Michael R. Hamblin, Ying-Ying Huang, Academic Press is an imprint of Elsevier, London, 2019; 529-544.
- Pasi M, Cordonnier Ch (2020) Clinical relevance of cerebral small vessel diseases. Stroke 51(1): 47-53.
- Maksimovich IV Transcatheter Treatment of Atherosclerotic Lesions of the Brain Complicated by Vascular Dementia Development 2012. World Journal of Neuroscience 2(4): 200-209.
- Hamblin MR (2019) Photobiomodulation for traumatic brain injury and stroke. J Neurosis Research 96(4): 731-743.
- Paro MR, Chakraborty AR, Angelo S, Nambiar S, Bulsara KR, et al. (2022) Molecular mediators of angiogenesis and neurogenesis after ischemic stroke. Rev Neurosci 34(4): 425-442.
- Radhakrishnan M, Kushwaha R, Acharya BS, Arvind KumarA, Chakravarty S, et al. (2024) Cerebral stroke-induced neurogenesis: Insights and therapeutic implications. Explor Neuroprot Ther 4: 172-197.
- Hachimi-Idrissi S (2023) Stem cell therapy in neurological disorders: Promises and concerns. Explor Neuroprot Ther 3: 346-362.
- Pendlebury ST, Rothwell PM (2019) Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based oxford vascular study. Lancet Neurol 18(3): 248-258.
- Regenhardt RW, Das AS, Lo EH (2018) Advances in understanding the pathophysiology of lacunar stroke: A Review. JAMA Neurol 75(10): 1273 1281.
- Caplan LR (2016) The effect of small artery disease on the occurrence and management of large artery disease. JAMA Neurol 73(1): 19-20.
- Akioka N, Takaiwa A, Kashiwazaki D, Kuwayama N, Endo Sh, et al. (2017) Clinical significance of hemodynamic cerebral ischemia on cognitive function in carotid artery stenosis: A prospective study before and after revascularization. J Nucl Med Mol Imaging. 61(3): 323-330.
- Haupert G, Ammi M, Hersant J, Tessot P, Papon X, et al. (2020) Treatment of carotid restenoses after endarterectomy: A retrospective monocentric study. Ann Vasc Surg 64: 43-53.
- Kim NY, Choi JW, Whang K, Cho SM, Koo YM, et al. (2019) Neurologic complications in patients with carotid artery stenting. J Cerebrovasc Endovasc Neurosurg. 21(2): 86-93.
- Yoo J, Choi JW, Lee SJ, Hong JM, Hong JH, et al. (2019) Ischemic diffusion lesion reversal after endovascular treatment. Stroke 50(6): 1504-1509.
- Gramegna LL, Cardozo A, Folleco E, Tomasello A (2020) Flow-diverter reconstruction of an intracranial internal carotid artery dissection during thrombectomy for acute ischaemic stroke. BMJ Case Rep 13(1): e231612.
- Snyder T, Agarwal S, Huang J, Ishida K, Flusty B, et al. (2020) Stroke treatment delay limits outcome after mechanical thrombectomy: Stratification by arrival time and ASPECTS. J Neuroimaging 30(5): 625-630.
- Yang Y, Guo D, Liu Y, Li Y (2024) Advances in neuroprotective therapy for acute ischemic stroke. Explor Neuroprot Ther 4: 55-71.
- Maksimovich IV (2019) Use of laser technologies in the treatment and rehabilitation of ischemic stroke in gerontological patients. Gerontol & Geriatric stud 5(3): 506-508.
- Hamblin MR (2018) Photobiomodulation, photomedicine and laser surgery: A new leap forward into the light for the 21st Photomed Laser Surg 36(8): 395-396.
- Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2): 199-212.
- Saltmarche AE, Margaret A, Naeser MA, Ho KF, Hamblin MR, et al. (2017) Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: Case series report. Photomedicine and Laser Surgery 35(8): 432-441.
- Salehpour F, Gholipour KS, Farajdokht, Kamari F, Walski T, et al. (2020) Therapeutic potential of intranasal photobiomodulation therapy for neurological and neuropsychiatric disorders: A narrative review. Reviews in the Neurosciences 31(3): 269-286.
- Hamblin MR. Mechanisms of photobiomodulation in the brain. Photobiomodulation in the Brain. Edited by Michael R. Hamblin, Ying-Ying Huang, Academic Press is an imprint of Elsevier, London, pp. 97-110.
- Lapchak PA. The challenge of effectively translating transcranial near-infrared laser therapy to treat acute ischemic stroke. Photobiomodulation in the Brain. Edited by Michael R. Hamblin, Ying-Ying Huang. Academic Press is an imprint of Elsevier, London, 2019: 289-298.
- Hennessy M, Hamblin MR (2017) Photobiomodulation and the brain: A new paradigm. Journal of Optics 19(1): 013003.
- Zhu Ch, Zhu X, Li H, Wang Sh, Shi N, et al. (2024) Recent advances in photodynamic therapy for vascular abnormalities. Photobiomodul Photomed Laser Surg 42(8): 501-508.
- Maksimovich IV (2019) Intracerebral transcatheter laser pbmt in the treatment of Binswanger’s disease and vascular parkinsonism: Research and clinical experience. Photobiomodul Photomed and Laser Surg 37(10): 606-614.
- Mester E, Szende B, Gartner P (1968) The effect of laser beams on the growth of hair in mice. Radiobiol Radiother (Berl) 9(5): 621-626.
- Deviatkov ND (1993) Application of electronics in medicine and biology. Electronic Equipment: Microwave Equipment 1(455): 66-76.
- Maksimovich IV (2004) Transluminal laser angioplasty in the treatment of ischemic brain lesions. Doctoral Dissertation, RUDN University: Peoples' Friendship University of Russia, Moscow.
- Tedford CE, DeLapp S, Jacques S, Anders J (2015) Quantitative analysis of transcranial and intraparenchymal light penetration in human cadaver brain tissue. Lasers Surg Med 47(4): 312–322.
- Johnson PK, Fino PC, Wilde EA, Hovenden ES, Russell HA, et al. (2024) The effect of intranasal plus transcranial photobiomodulation on neuromuscular control in individuals with repetitive head acceleration events. Photobiomodul Photomed Laser Surg 42(6): 404-413.
- Ming WL, Chia HY, Pi Yu Sung, Sen Wei Tsai (2022) Intravascular laser irradiation of blood improves functional independence in subacute post-stroke patients: a retrospective observational study from a post-stroke acute care center in taiwan. Photobiomodulation, Photomedicine and Laser Surgery 40(10): 691–697.
- Maksimovich IV (2016) Transcatheter cerebral revascularization in the treatment of atherosclerotic lesions of the brain. Brain Disorders & Therapy 5(1): 1-8.
- Mahoney FI, Barthel DM (1965) Functional evaluation: The barthel index. Maryland State Medical Journal 14: 61-65.
- Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr. Res 12(3): 189-198.
- Morris JC (1993) The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 43(11): 2412-2414.
- Maksimovich IV (2023) Cerebral small vessel disease in neurodegenerative lesions. Explor Res Hypothesis Med 9(3): 258-260.
- Maksimovich IV (2006) Method for carrying out transluminal laser-induced brain revascularization in atherosclerotic injury cases. Patent RU, Moscow, Russia.
- Maksimovich IV (2008) Method of transluminal laser revascularization of cerebral blood vessels having atherosclerotic lesions. Patent US, 7490612.
- Maksimovich IV (2022) Study of the impact of transcatheter intracerebral laser photobiomodulation therapy treatment on patients with Alzheimer's Disease and Binswanger's Disease. Medical Research Archives 10(12): 1-13.
© 2024 Ivan V. Maksimovich. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






