- Submissions

Full Text
Experimental Techniques in Urology & Nephrology
Renal Function Markers in Rheumatoid Arthritis: A Case-Control Study from Shendi City
Yageen Kamal Abdalrazig Ahmed1, Nazar Alsir1, Abdelwahab Abdien Saeed1, Tibyan Abdalmajed Altaher1, Ghanem Mohammed Mahjaf2, Rahma Abdo Ahmed Osman1 and Mosab Nouraldein Mohammed Hamad3*
1 Department of Clinical Chemistry, Faculty of Medical Laboratory Sciences, Shendi University, Sudan
2Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, Shendi University, Sudan
3 Assistant Professor, Microbiology Department, Faculty of Medicine, Elsheikh Abdallah Elbadri University, Sudan
*Corresponding author: Mosab Nouraldein Mohammed Hamad, Microbiology department, Faculty of Medicine, Elsheikh Abdallah Elbadri University, Sudan
Submission: September 09, 2025;Published: November 06, 2025
ISSN 2578-0395Volume3 Issue3
Abstract
Background: Patients with rheumatoid arthritis face a higher risk of developing chronic kidney disease
and glomerulonephritis, which increases mortality, with a hazard ratio (HR) of 2.77-4. 45.
Objective: This study aims to assess urea and creatinine levels in patients with rheumatoid arthritis.
Materials and methods: This cooperative study was conducted in Shendi City, involving a total
sample of 50 individuals, including 30 patients with rheumatoid arthritis as the case group and 20
healthy individuals as the control group. Serum urea and creatinine levels were estimated using a
spectrophotometer biosystem.
Results: This study showed that the levels of urea and creatinine were significantly increased in
rheumatoid arthritis patients, with case levels at (89.43 ± 51.949) and control levels at (38. 38.60 ±
5.707) (P value=0.000) for urea and (3.3529 ± 3.830) for creatinine in patients with rheumatoid arthritis,
with case levels at (3.830 ± 3.3529) and control levels at (0.695 ± 0.1905) (P value = 0. 000). There was
a significant effect of urea levels on age (0.783, P=0.003). A weak association was observed between
creatinine and age (P=0.910). Males exhibited higher levels of both urea and creatinine compared to
females, with urea levels being significantly more affected (P value=0.019) than creatinine levels (P
value=0.343). A significant difference was noted between the groups with and without a family history
of RA (P values of 0.012 and 0.480, respectively). NSAIDs were significantly more effective than the non-
NSAID group in lowering urea and creatinine levels (P values=0.000 and 0.003, respectively).
Conclusion: Patients with rheumatoid arthritis have higher serum levels of creatinine and urea. Many
patients with rheumatoid arthritis frequently experience renal issues and their elevated urea levels
are closely associated with their age and gender. The burden of chronic kidney illness among research
participants may be alleviated by effectively managing positive cases and early screening of RA patients
for renal disorders. Screening for renal abnormalities could help patients with rheumatoid arthritis
prevent renal complications and assess their risk early
Keywords: Rheumatoid arthritis; Creatinine; Urea; Sudanese; Chronic kidney disease
Introduction
Rheumatoid Arthritis (RA) is an autoimmune disorder that causes chronic inflammation of the joints. Autoimmune diseases occur when the body’s tissues are mistakenly attacked by the body’s immune system. The immune system consists of a complex organization of cells and antibodies designed to “seek and destroy” invaders, particularly infections. Patients with autoimmune diseases have antibodies and immune cells in their blood that target their body tissues, leading to inflammation. While inflammation of the tissue around the joints and inflammatory arthritis are characteristic features of rheumatoid arthritis, the disease can also cause other symptoms of RA [1]. The cause of rheumatoid arthritis is believed to be a combination of genetic and environmental factors. A family history of rheumatoid arthritis increases the risk approximately three to five times. Smoking, belonging to the Caucasian population and other factors also increase the risk of rheumatoid arthritis. Periodontal disease has been associated with rheumatoid arthritis, although infectious agents such as viruses, bacteria and fungi have long been suspected. The disease progresses from phase 1, where there is non–specific inflammation, to phase 2, characterized by amplification in the synovium and then to phase 3, or chronic inflammation [2]. Rheumatic disease and kidney disease are both common in the general population. Rheumatologists are frequently exposed to patients with concomitant renal disease. In fact, 18% of patients in rheumatology clinics have a Glomerular Filtration Rate (GFR) of 60ml/minute or less, compared to 5% in the general population. When patients present with both arthritis and kidney disease, the following questions must be addressed: Is kidney disease a complication of rheumatic disease or its management, or are they both manifestations of a single systemic autoimmune disease? This review addresses these questions and may help attending specialists, either rheumatologists or nephrologists, manage patients with concomitant rheumatic disease and kidney disease [1]. RA can affect the kidneys either directly due to the disease itself or secondarily from the drugs used for treatment, including biological and non-biological Disease-Modifying Anti- Rheumatic Drugs (DMARDs) [3-5], or analgesics like Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). It has been found that renal disease is common in patients with rheumatoid arthritis based on regular assessments of serum and urine parameters of renal function, leading to the conclusion that patients with rheumatoid arthritis must be routinely monitored through blood and urinary parameters for concomitant chronic kidney disease (CKD) [6]. Early intervention in the disease may reduce the risk of kidney involvement and the potential decrease in eGFR [7]. The decline of eGFR in RA patients can be slowed by using biologic DMARDs, which in turn helps to delay the progression of CKD in these individuals [8]. Furthermore, effective management of CKD progression in RA may contribute to lowering cardiovascular complications and mortality rates within this group [9]. The objective of this study is to evaluate serum urea and creatinine levels in Sudanese patients with rheumatoid arthritis. Recent evidence suggests that the anti-aging gene Sirtuin 1 (SIRT1) plays a vital role in reducing inflammation, oxidative stress and tissue damage associated with chronic diseases, including rheumatoid arthritis and chronic kidney disease. SIRT1 activation promotes cellular protection, while its inactivation has been linked to multiple organ dysfunction and accelerated disease progression. The dysregulation of SIRT1 expression may therefore contribute to renal impairment in patients with rheumatoid arthritis. Assessing SIRT1 levels may help identify patients at higher risk of developing renal complications [10-13].
Methodology
Study design
This is a descriptive case-control study conducted from January to April 2025, aimed at assessing urea and creatinine levels in patients with rheumatoid arthritis.
Study area
Different hospitals and clinical centers are located in Shendi locality, River Nile State, Sudan. Shendi is a town in Northern Sudan, situated on the east bank of the Nile, 150km northeast of Khartoum. It is also about 45km southwest of the ancient city of Meroe. Located in the River Nile State, Shendi is the center of the Ja’aliin tribe and an important historic trading center. Its principal suburb on the west bank is Al-Matamma. A major traditional trade route across the Bayuda desert connects Al-Matamma to Marawi and Napata, 250km to the northwest.
Study population
The study population consists of 30 patients with rheumatoid arthritis and 20 healthy subjects serving as controls.
Inclusion criteria
Patients with rheumatoid arthritis and healthy subjects as controls were included.
Exclusion criteria
Infectious diseases, the use of specific medications, metabolic diseases, a family history of certain endocrinopathies, liver disease and other chronic conditions.
Study sample
5ml of venous blood was collected from patients in a plain container (a container without anticoagulant).
Sample size
A total of 30 patients with rheumatoid arthritis formed the test group, while 20 healthy individuals served as the control group.
Study variable
Including age, gender, diagnosis, family history and medication.
Tools of data collection
Data was collected via a questionnaire, which was designed to provide personal and medical information about the subject.
Data analysis
After examination of the sections, the results of the laboratory investigation, as well as the demographic data from the patient’s records, were processed using the Statistical Packages for Social Sciences (SPSS) computer program. Frequency, mean and chisquare test values were calculated at <0.05 and considered statistically significant.
Results
(Tables 1-7)
Table 1: Comparison between the case and control groups based on urea levels.
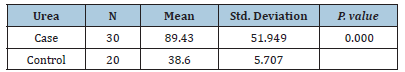
Table 2: Comparison between Case and Control based on Creatinine levels
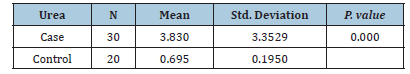
Table 3: The effect of Age on the Urea & Creatinine
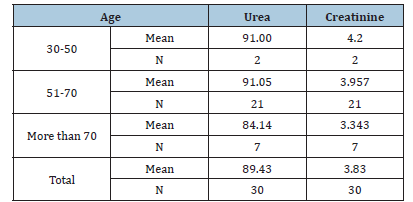
Table 4: The effect of Gender on the Urea and creatinine
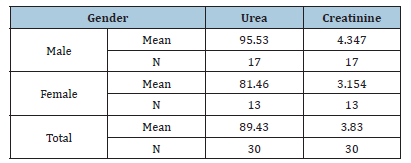
Table 5: The effect of Diagnosis on the Urea and creatinine
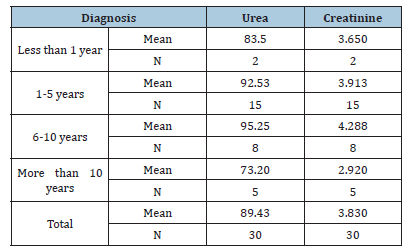
Table 6: The effect of Family History on the Urea & Creatinine
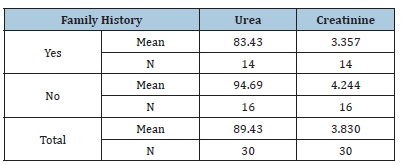
Table 7:The effect of medication on the urea & creatinine
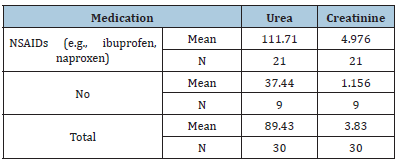
Discussion
RA is a chronic autoimmune disease characterized by inflammation that affects various tissues in the body and leads to a range of adverse effects, including joint destruction and, potentially, patient death. Renal disorders have been reported in RA patients, resulting in symptoms such as proteinuria, hematuria and renal dysfunction in some cases [14,15]. Renal failure not only complicates RA management but also elevates the risk of death among these patients [16]. A case-control study conducted from February to April 2025 in Shendi locality found that Rheumatoid Arthritis (RA) is an autoimmune disease that causes a chronic, systemic inflammatory disorder affecting multiple tissues and organs, primarily targeting flexible (synovial) joints. It can be a disabling and painful condition, leading to significant loss of functioning and mobility if not adequately treated [1]. This study aimed to measure urea and creatinine levels among patients with rheumatoid arthritis. The results revealed a significant increase in the mean levels of urea and creatinine in the cases compared to the control group, with p-values of 0.000 and 0.000, respectively. This finding aligns with studies conducted by Koseki and his colleagues [14]. Furthermore, research by Anders et al. indicated a significant increase in urea and creatinine levels in RA cases compared to the control group [3]. The mean± SD of serum urea and creatinine levels were significantly higher in RA patients than in healthy individuals, consistent with a study by Ansari [17] involving 100 RA patients at the Department of Biochemistry, Govt. Medical College, Jalaun, Orai, which showed significantly elevated serum urea and creatinine levels (p<0.01) in untreated RA patients versus healthy controls. Based on these observations, he concluded that elevated serum urea and creatinine levels are associated with rheumatoid arthritis and various kidney disorders, which agrees with the current study [17]. This finding contrasts with the study conducted by Haroon and colleagues, who found no significant difference in means between RA patients and those with seronegative arthritis (83 vs. 97mL/ min/1.73 m2) [18]. According to the findings of this study, urea and creatinine levels are higher in individuals aged 30-50 years, with a 24.3% prevalence for those over 40, compared to other age groups [19]. Saisho et al. reported a prevalence of Chronic Kidney Disease (CKD) in RA patients based on eGFR as 25.4% in G1 stage, 55.9% in G2 stage, 17.5% in G3 stage, 0.8% in G4 stage and 2.2% in G5 stage [20]. In addition to these findings, emerging molecular studies highlight the role of Sirtuin 1 (SIRT1) as a protective factor in both rheumatoid arthritis and renal disease. SIRT1 regulates cellular metabolism, inflammation and apoptosis and its activation has been shown to reduce disease severity in autoimmune and renal disorders. Inactivation of SIRT1 can promote oxidative stress and tissue injury, leading to multiple organ dysfunction. Therefore, future studies should evaluate plasma SIRT1 levels in RA patients to determine their correlation with renal dysfunction and disease activity. These findings could provide a molecular insight into the pathophysiological mechanisms linking RA and CKD. In the present study, NSAIDs demonstrated significantly greater effectiveness than the non-NSAID group in reducing urea and creatinine levels (P values=0.000 and 0.003, respectively). The findings from Onishi et al. indicated that the effectiveness of biological drugs in slowing eGFR decline and thus reducing CKD risk is significantly higher than that of other drugs used for RA treatment [21]. A study on hydroxychloroquine (HCQ) reported CKD in 3.96% of users compared to 8.59% of non-users, suggesting a lower incidence of CKD among HCQ users [22].
Conclusion
Patients with rheumatoid arthritis exhibit higher serum levels of creatinine and urea. They often experience renal issues, with elevated urea levels closely linked to their age and gender. Aggressively managing positive cases and conducting early screenings for renal disorders in RA patients may alleviate the burden of chronic kidney illness among research participants. Furthermore, evaluating SIRT1 levels may serve as an additional biomarker for predicting renal dysfunction among rheumatoid arthritis patients. Early screening for renal abnormalities could help patients with rheumatoid arthritis avoid renal consequences and better assess their risk.
Limitations
While this is the first research from Shendi City, Sudan, focusing on renal impairment in RA patients, it is still a single-center study with a limited sample size.
Consent
The patient’s written consent has been collected.
Ethical Approval
The study was approved by the Department of Clinical Chemistry in Medical Laboratory Sciences at Shendi University and reviewed by the ethics committee board. Sample collection took place after participants signed a written agreement. Permission for this study was obtained from the local authorities in the study area. The aims and benefits of this study were explained, along with an assurance of confidentiality. All protocols in this study were conducted in accordance with the Declaration of Helsinki (1964).
Sources of Funding
There was no specific grant for this research from any funding organization in the public, private, or nonprofit sectors.
Conflict of Interest
The authors have declared that no competing interests exist.
References
- Hill AJ, Thomson RJ, Hunter JA, Traynor JP (2009) The prevalence of chronic kidney disease in rheumatology outpatients. Scottish Medical Journal 54(2): 9-12.
- Murtaza M (2018) Clinical advancement in rheumatoid arthritis. IOSR J Dent Med Sci 17(2): 55-61.
- Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncol 11(6): 694-703.
- Durando M, Tiu H, Kim JS (2017) Sulfasalazine-induced crystalluria causing severe acute kidney injury. Am J Kidney Dis 70(6): 869-873.
- Braun A, Zeier M, (2004) Rheumatoid arthritis and the kidney: Uneasy companions. Nephron Clin Pract 96(4): c105-c106.
- Widemann BC, Adamson PC (2006) Understanding and managing methotrexate nephrotoxicity. Oncol 11(6): 694-703.
- Kapoor T, Bathon J (2018) Renal manifestations of rheumatoid arthritis. Rheum Dis Clin North Am 44(4): 571-584.
- Sumida K, Molnar MZ, Potukuchi PK, Hassan F, Thomas F, et al. (2018) Treatment of rheumatoid arthritis with biologic agents lowers the risk of incident chronic kidney disease. Kidney Int 93(5): 1207-1216.
- Chiu HY, Huang HL, Li CH, Chen HA, Yeh CL, et al. (2015) Increased risk of chronic kidney disease in rheumatoid arthritis associated with cardiovascular complications - a national population-based cohort study. Plos One 10(9): e0136508.
- Ian James Martins (2016) Anti-aging genes improve appetite regulation and reverse cell senescence and apoptosis in global populations. Advances in Aging Research 5(1): 9-26.
- Ian James Martins (2017) Single gene inactivation implications to diabetes and multiple organ dysfunction syndrome. Journal of Clinical Epigenetics 3(3): 24.
- Ian James Martins (2017) Nutrition therapy regulates caffeine metabolism with relevance to NAFLD and induction of type 3 diabetes. Journal of Diabetes and Metabolic Disorders 4: 019.
- Ian James Martins (2018) Sirtuin 1, a diagnostic protein marker and its relevance to chronic disease and therapeutic drug interventions. EC Pharmacology and Toxicology 6(4): 209-215.
- Koseki Y, Terai C, Moriguchi M, Uesato M, Kamatani N, et al. (2001) A prospective study of renal disease in patients with early rheumatoid arthritis. Annals of the Rheumatic Diseases 60(4): 327-31.
- Karie S, Gandjbakhch F, Janus N, Launay-Vacher V, Rozenberg S, et al. (2008) Kidney disease in RA patients: Prevalence and implication on RA-related drugs management: The MATRIX study. Rheumatology 47(3): 350-354.
- Sihvonen S, Korpela M, Mustonen J, Laippala P, Pasternack A, et al. (2004) Renal disease as a predictor of increased mortality among patients with rheumatoid arthritis. Nephron Clinical Practice 96(4): c107-c114.
- Ansari SK (2016) NSAIDs and renal function in rheumatoid arthritis. Journal of Medical Science and Clinical Research 4(2): 9412-9416.
- Haroon M, Adeeb F, Devlin J, Gradaigh DO, Walker F, et al. (2011) A comparative study of renal dysfunction in patients with inflammatory arthropathies: Strong association with cardiovascular diseases and not with anti-rheumatic therapies, inflammatory markers or duration of arthritis. Int J Rheum Dis 14(3): 255-260.
- Bouya S, Balouchi A, Rafiemanesh H, Hesaraki M (2018) Prevalence of chronic kidney disease in Iranian general population: A meta‐analysis and systematic review. Therapeutic Apheresis and Dialysis 22(6): 594-599.
- Saisho K, Yoshikawa N, Sugata K, Hamada H, Tohma S, et al. (2016) Prevalence of chronic kidney disease and administration of RA-related drugs in patients with RA: The NinJa 2012 study in Japan. Modern Rheumatology 26(3): 331-335.
- Onishi A, Akashi K, Yamamoto W, Ebina K, Murata K, et al. (2021) The association of disease activity and estimated GFR in patients with rheumatoid arthritis: Findings from the Answer study. American Journal of Kidney Diseases 78(5): 761-764.
- Wu CL, Chang CC, Kor CT, Yang TH, Chiu PF, et al. (2018) Hydroxychloroquine use and risk of CKD in patients with rheumatoid arthritis. Clinical Journal of the American Society of Nephrology 13(5): 702-709.
© 2021 Mosab Nouraldein Mohammed Hamad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






