- Submissions

Full Text
Experiments in Rhinology & Otolaryngology
The Relationship Between Sleep Apnea Syndrome and Ascending Aorta Diameter
Ahmad Huraibat1, Helen Bornaun2*, Nazlican Civilibal Tang3, Savas Dedeoğlu4, Salih Salihi5 and Deniz Bornaun6
1Department of Cardiology, Istanbul Saglik Bilimleri University Kanuni Sultan Suleyman Training and Research Hospital, Turkey
2Department of Pediatric Cardiology, Istanbul Saglik Bilimleri University Kanuni Sultan Suleyman Training and Research Hospital, Turkey
3Department of Pediatrics, Istanbul Saglik Bilimleri University Kanuni Sultan Suleyman Training and Research Hospital, Turkey
4Department of Pediatric Cardiology, Uskudar University, Turkey
5Department of Cardiovascular Surgery, Sakarya University Training and Research Hospital, Turkey
6Faculty of Medicine, Istanbul University, Turkey
*Corresponding author: Helen Bornaun, Department of Pediatric Cardiology, Istanbul Saglik Bilimleri University Kanuni Sultan Suleyman Training and Research Hospital, Turkey
Submission: February 09, 2023;Published: February 20, 2023

ISSN 2637-7780 Volume3 Issue3
Abstract
Objective: Obstructive Sleep Apnea Syndrome (OSAS) is known to be associated with cardiovascular
disorders such as hypertension, coronary artery disease, and arrhythmia. However, only a few studies
have examined its role in aortic dilatation. We aimed to determine the relationship between obstructive
sleep apnea syndrome and aortic diameter.
Methods: 53 patients (42 males, 11 females) being of age between 20 and 80 years, who presented to
the chest diseases outpatient clinic with symptoms compatible with sleep apnea syndrome included this
study. Berlin Sleep Questionnaire and Epworth Sleepiness Scale were used to determine the OSAS risk.
Following risk assessment, polysomnography test was performed to confirm OSAS. Apnea-Hypopnea
Index (AHI)<5 was considered normal and AHI≥5 was considered to be consistent with OSAS. They were
informed about OSAS and its adverse effects on cardiovascular system. Transthoracic echocardiography
was used as a noninvasive diagnostic tool to quantify proximal aorta diameter in order to detect aortic
dilatation.
Results: 40 people were diagnosed with OSAS by polysomnography. 13 subjects did not have OSAS.
Obstructive Sleep Apnea Syndrome (OSAS) group had significantly greater sinotubular junction and
ascending aorta diameters (p<0.05). Sinus valsalva dilatation was more common among the OSAS group,
although this difference did not reach statistical significance.
Conclusion: A positive correlation was found between Apnea-Hypopnea Index (AHI) and aortic
dilatation. Early diagnosis and effective treatment of obstructive sleep apnea is important to prevent
aortic complications.
Keywords:Sleep apnea; Aortic dilatation; Proximal aortic diameter
Introduction
Obstructive Sleep Apnea Syndrome (OSAS) is a clinical condition characterized by episodes of respiratory apnea during sleep, sleep fragmentation, oxygen desaturation, daytime sleepiness, and sudden episodes of falling asleep [1]. Its role in traffic accidents and workforce loss, in addition to its already known cardiovascular and neurological complications, has recently made it a subject of intensive research [2,3]. The main cardiovascular disorders complicating obstructive sleep apnea are Hypertension (HT), Coronary Artery Disease (CAD), Metabolic Syndrome (MS), cardiac arrhythmias, left Heart Failure (HF), pulmonary hypertension, right heart failure, polycythemia, stroke, and sudden death. OSAS affects 50% of patients with hypertension, 25% of patients with chronic heart failure, 30% of patients with acute coronary syndrome, and 60% of patients with stroke [4-6]. Increased negative intrathoracic pressure induced by inspiratory effort during repetitive apnea periods at sleep exerts a mechanical stress on aortic wall, contributing to the emergence and progression of aortic disorders such as aortic aneurysm and dissection. It has been reported that this may also be related to intermittent hypoxia and reoxygenization [7]. Many studies to date have investigated the relationship between, OSAS, hypertension and coronary artery disease. Herein, we aimed to study the relationship between obstructive sleep apnea syndrome and aortic diameter.
Method
This study involved a total of 53 patients who presented to the chest diseases outpatient clinic with symptoms compatible with sleep apnea syndrome between January 2015 and April 2016. Berlin Sleep Questionnaire and Epworth Sleepiness Scale were used to determine the OSAS risk [8]. Following risk assessment, polysomnography test was performed to confirm OSAS. The study protocol was reviewed and approved by the Ethics Committee (No: 2014/5 and Date: 20/06/2014). Written informed consent was obtained from all the participants. All patients were admitted to the hospital for at least 8 hours and they slept for at least 4 hours for the polysomnography test, which was used to obtain the following data: apnea hypopnea index and oxygen desaturation index, EEG, ECG, EMG, chest, abdomen, eye, and jaw motion sensor recordings and snoring sensor recordings. Patients who underwent polysomnography after enrollment were informed about OSAS and its adverse effects on cardiovascular system. Transthoracic echocardiography was used as a noninvasive diagnostic tool to quantify proximal aorta diameter in order to detect aortic dilatation. The inclusion criteria were being diagnosed with OSAS by polysomnography and being of age between 20 and 80 years. Patients with a previous history of aortic disease including aneurysm, dissection, or cardiac surgery, connective tissue disorders known to cause aortic dilatation or aneurysm (e.g Marfan, Behcet, Ehlers Danlos syndrome) and severe infection were excluded. All enrolled patients had their demographic characteristics recorded. Body Mass Index (BMI) and Body Surface Area (BSA) were calculated using the following formulae: BMI=(Weight kg) / (Height cm)² and BSA= (Weight 0.425 x Height 0.725) x 0.007184. History of hypertension, diabetes, hyperlipidemia, coronary artery disease, heart failure, and smoking were recorded. Systolic and diastolic blood pressures and heart rhythm were recorded. The Apnea Hypopnea Index (AHI) values obtained from polysomnography were interpreted according to (Table 1). AHI<5 was considered normal and AHI≥ 5 was considered to be consistent with OSAS.
Table 1:The severity of OSAS based on the AHI.

Echocardiography
All echocardiographic examinations were performed with Vivid-7 PRO echocardiography device using M4S 2.0MHz harmonic cardiac transducer, with patients in the left lateral decubitus position. Prior to the procedure, blood pressure and heart rate were recorded. The measurements were done in compliance with the “Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging” published by Lang RM et al. [9]. Two dimensional and M-mode images from parasternal short-axis windows were used to record LVEDD and LVESD. Left atrial diameter was measured perpendicular to aortic root at the end of left ventricular systole while the aortic valves were maximally open in parasternal short axis window. Interventricular Septal thickness (IVS): interventricular septal thickness was measured approximately 2cm below the aortic valve at end-diastole when the mitral valve was open using 2-D echocardiography in parasternal long-axis window. Left Ventricular Ejection Fraction (LVEF) was quantified by the modified Simpson’s method from the apical 4-chamber view. Maximal values of mitral E and A waves were measured to calculate the E/A ratio.
Figure 1:Junction diameter; AscAo: Ascending aorta diameter.
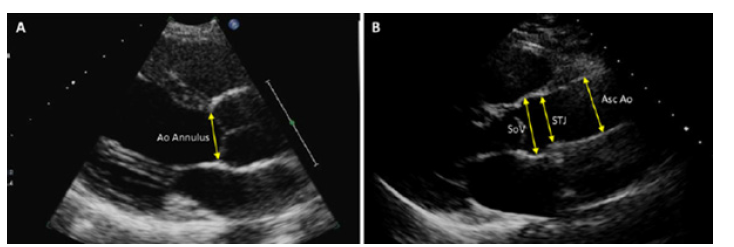
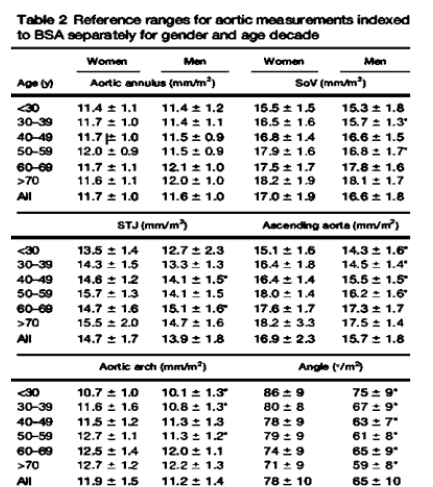
Proximal aortic diameter was recorded from 4 separate sites as described below (Figure 1). The diameters of sinus of valsalva, sinotubular junction and ascending aorta were quantified at enddiastole from the leading edge to leading edge technique using 2-D echocardiography in the parasternal long-axis window. The annular diameter was recorded between the tips of the right cusp and non-coronary aortic cusps from the inner edge to inner edge at mid-systole. Normal aortic reference values were determined on the basis of sex, BMI, and age for all patients according to previously published data (Figure 2) [10]. All images were taken and recorded by a cardiologist participating in the study who applied the same protocol for all patients.
Figure 2:Normal aortic reference diameters determined by age, sex, and BMI.
Statistical analyses
All statistical analyses were carried out using Windows XP-SPSS 2007 statistical software. The descriptive statistics included mean, standard deviation, and frequency. Comparison of quantitative variables was done with Mann Whitney-U test. Comparison of normally distributed qualitative variables was done with Chi- Square test. Pearson correlation analysis was used to test possible correlations between normally distributed variables. A p value of less than 0.05 was considered statistically significant for all statistical analyses.
Results
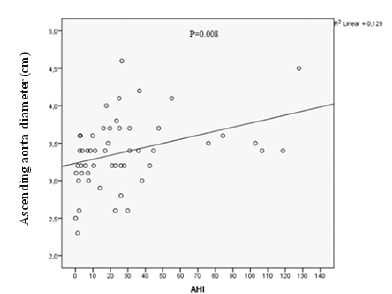
This study involved a total of 53 patients who had symptoms of obstructive sleep apnea and presented to Department of Chest Diseases between January 2015 and April 2016. 42 (79.2%) were men and 11 (20.8%) were women. The participants were divided into 2 groups; one of which contained 40 patients diagnosed with OSAS by polysomnography and 13 subjects who were free of OSAS. 80% were male in the OSAS group and 76.9% were male in the control group, with the two groups having a similar gender distribution (P=1.00). They were comparable in terms of the frequency of comorbidities. The OSAS group had a higher rate of hypertension, coronary artery disease, heart failure, and COPD, albeit it did not reach statistical significance because of lower number of cases (Table 2 & 3). Summarizes the findings of physical examination in both groups. The groups did not significantly differ from each other in regard to blood pressure and pulse rate (p˃0.05). The echocardiographic findings were shown on (Table 4). OSAS group had significantly greater sinotubular junction and ascending aorta diameters (p<0.05). Sinus valsalva dilatation was more common among the OSAS group, although this difference did not reach statistical significance (P=0.74) (Table 4). AHI was significantly and positively correlated with systolic blood pressure (P=0.004), diastolic blood pressure (P=0.001), BMI (P=0.000), IVS thickness (P=0.005) and ascending aorta diameter (P=0.008). However, other echocardiographic findings were not significantly correlated with AHI (Table 5).
Table 2:Comparison of the demographic characteristics of the study groups.

(BMI: body mass index, BSA: body surface area, AHI: Apnea-Hypopnea İndex, DM: diabetes mellitus, HT: Hypertension, CAD: Corornary Artery Disease, HF: Heart Failure, COPD: Chronic obstructive pulmonary disease)
Table 3:Comparison of physical examination findings of the study groups.

Table 4:Comparison of the echocardiographic findings of the study group.

Table 5:Correlation of various parameters with AHI.

Discussion
Obstructive Sleep Apnea Syndrome (OSAS) is known to be associated with cardiovascular disorders [4] such as hypertension, coronary artery disease, and arrhythmia [4,6-7]. However, only a few studies have examined its role in aortic dilatation. The aim of our study was to determine the prevalence of aortic dilatation in patients who presented with symptoms of sleep apnea syndrome. Several mechanisms are thought to play a role in the pathophysiology of aortic dilatation in OSAS. One of these mechanisms is increased nocturnal blood pressure and worsening aortic dilatation as a result of increased sympathetic nervous system activity at night. Another mechanism involves an increased mechanical stress on the aortic wall which results from an increased intrathoracic pressure created by inspiratory effort to overcome upper airway obstruction.
In the current study, OSAS patients had significantly greater proximal aortic diameters including aortic annulus and sinotubular junction, and particularly sinus valsalva and ascending aorta when compared to the control subjects (Figure 3). Disorders potentially linked to aortic dilatation (e.g. hypertension, coronary artery disease) and other chronic disorders associated with OSAS (heart failure, diabetes mellitus, COPD) were not significantly more common in the OSAS group. Serizawa N et al. [11] investigated the relationship between OSAS and aortic dilatation in 150 patients. In their study, OSAS, defined as AHI≥10/h, was diagnosed in 73% of patients. Thoracic aorta diameter measured by Computerized Tomography (CT), was positively correlated to AHI(P<0.001) (Figure 4). Similar to their findings, we also found that aortic dilatation was correlated to sleep apnea syndrome. Their study also showed that age and male gender were correlated to aortic dilatation. Although CT provides more accurate aortic diameter measurements than transthoracic echocardiography, it has some important disadvantages such as radiation exposure, cost and contrast nephropathy; thus, studies using echocardiography are safer for the patients. I another study by Baguet JP et al. [12], it was reported that echocardiographically determined diameter of the aortic root was increased in 156 patients with newly diagnosed OSAS who were free of any cardiac disorder. They also reported that aortic diameter was positively correlated with age and blood pressure. The authors suggested that aortic root diameter was dependent on diastolic blood pressure but not on AHI. Their patients were obese with a mean BMI of 27±3 kg/m², and it is already known that BMI is an independent determinant of aortic diameter. An increase in aortic diameter detected by our study was consistent with the findings of their study. Unlike their study, we quantified aortic diameter from 4 separate sites (annulus, sinus valsalva, sinotubular junction, and ascending aorta). The subgroup analyses revealed that the correlations between those 4 distinct diameters and OSAS were different from one another.
Figure 3:Prevalences of ascending aorta and sinus valsalva dilatation of both groups.
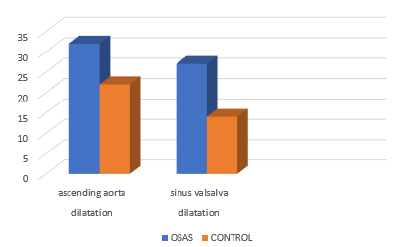
Figure 4:The scatter diagram of the correlation analysis between AHI and aorta diameter.
Lee LC et al. [13], studied the relationship between OSAS and thoracic aorta diameter in a cohort of men who were at the subacute phase of acute Myocardial Infarction (MI). They found a positive correlation between thoracic aorta diameter and age, hypertension, and BMI, but failed to demonstrate a significant correlation between OSAS and aortic dilatation. Their study enrolled only men who sustained a myocardial infarction. High incidence of comorbidities in their study such as hyperlipidemia (84%), diabetes mellitus (39%), hypertension (51%) and male gender are also known to be the risk factors of aortic dilatation and aneurysm [14]. Therefore, we believe that they must have influenced the study results. Our study, on the other hand, included patients of both genders who were free of aorta or acute cardiovascular disorders. In contrast to theirs, our study showed a significant correlation between thoracic aortic diameter and OSAS.
Kohler M et al. [15], examined the relationship between OSAS and aortic diameter in 61 patients with Marfan syndrome. They reported a positive correlation between OSAS severity and aortic diameter, indicating that OSAS is an independent risk factor for aortic dilatation in patients with Marfan syndrome. Although we demonstrated that the OSAS patients without Marfan syndrome had a greater proximal aortic diameter than the control subjects, this difference did not reach statistical significance due to the small sample volume of our study.
In a patient with a definitive diagnosis of sleep apnea syndrome, it is still unclear and debated whether the pathophysiology of aortic dilatation is an OSAS-induced increase in intrathoracic pressure that exerts a tensile force on aortic wall with augmented aortic distention or it simply occurs as a result of a hypertension-induced mechanical stress on aorta, which usually accompanies OSAS. The common point of all previous studies on this subject is that they provide evidence of a relationship between OSAS and aortic diameter. Although there exist multiple studies scrutinizing the link between OSAS and cardiovascular disease, only a few studies have specifically examined the possible link between OSAS and aortic dilatation or aneurysm. No study has yet investigated how positive airway pressure affected the change in aortic diameter, need for surgery or mortality. Further studies are needed in this field.
Conclusion
A positive correlation was found between AHI and aortic dilatation. Early diagnosis and effective treatment of obstructive sleep apnea is important to prevent aortic complications. The follow-up of the patients are still ongoing, and we intend to investigate whether C-pap treatment has any favorable impact on aortic diameter.
Limitations of the Study
One of the main limitations of the present study is the small sample volume of the study population in both groups, a limitation that directly affected its statistical analyses. In order to determine the prevalence of aortic dilatation in OSAS more accurately, studies with larger sample size are needed. Aortic quantification by echocardiography is highly accurate and close to actual values but is less sensitive compared to computerized tomography.
Declaration of Conflicting Interests
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Acknowledgement
The authors received no financial support for the research and/ or authorship of this article.
References
- Strohl KP, Redline S (1996) Recognition of obstructive sleep apnea. Am J Respir Crit Care Med 154: 279-289.
- Partinen M, Jamieson A, Guilleminault C (1988) Long-term outcome for obstructive sleep apnea syndrome patients: Mortality. Chest 94(6): 1200-1204.
- Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB (1996) Investigating the relationship between stroke and obstructive sleep apnea. Stroke 27(3): 401-407.
- Lattimore JD, Celermajer DS, Wilcox I (2003) Obstructive sleep apnea and cardiovascular disease. J Am Coll Cardiol 41(9): 1429-1437.
- Gillon JCM, Treacher DF, Gaminara EJ, Pearson TC, Cameron IR (1986) Intermittent hypoxia in patients with unexplained polycythaemia. Br Med J 293(6547): 588-590.
- Javaheri S (2006) Sleep disorders in systolic heart failure: A prospective study of 100 male patients. The final report. Int J Cardiol 106(1): 21-28.
- Fava C, Montagnana M, Favaloro EJ, Guidi GC, Lippi G (2011) Obstructive sleep apnea syndrome and cardiovascular diseases. Semin Thromb Hemost 37(3): 280-297.
- Lawrance KS (1990) The international classification of sleep disorders: Diagnostic and coding manual. ASDA, pp. 65-71.
- Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28(1): 1-39.
- Mirea O, Maffessanti F, Gripari P, Tamborini G, Muratori M, et al. (2013) Effects of aging and body size on proximal and ascending aorta and aortic arch: Inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J Am Soc Echocardiogr 26(4): 419-427.
- Serizawa N, Yumino D, Takagi A, Gomita K, Kajimoto K, et al. (2008) Obstructive sleep apnea is associated with greater thoracic aortic size. J Am Coll Cardiol 52(10): 885-886.
- Baguet JP, Minville C, Tamisier R, Roche F, Rochette GB, et al. (2011) Increased aortic root size is associated with nocturnal hypoxia and diastolic blood pressure in obstructive sleep apnea. Sleep 34(11): 1605-1607.
- Lee LC, Torres MC, Khoo SM, Chong EY, Lau C, et al. (2010) The relative impact of obstructive sleep apnea and hypertension on the structural and functional changes of the thoracic aorta. Sleep 33(9): 1173-1176.
- Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, et al. (2010) Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 52(3): 539-548.
- Kohler M, Blair E, Risby P, Nickol AH, Wordsworth P, et al. (2009) The prevalence of obstructive sleep apnoea and its association with aortic dilatation in marfan’s syndrome. Thorax 64(2): 162-166.
© 2023 Helen Bornaun. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






