- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Primary Progressive Aphasia: Clinical Approach and Psychosocial Needs
Letteria Tomasello*
Department of Clinical and Experimental Medicine, University of Messina, Italy
*Corresponding author:Letteria Tomasello, Department of Clinical and Experimental Medicine, University of Messina, Italy
Submission: July 30, 2025;Published: September 03, 2025

ISSN 2637-7934 Volume5 Issue3
Abstract
Primary Progressive Aphasia (PPA) is a cognitive impairment that involves a progressive loss of language function. Language is a human faculty that allows us to communicate with each other. Our language functions include speaking, understanding what others are saying, repeating things we have heard, naming common objects, reading and writing. “Aphasia” is a term used to refer to deficits in language functions. PPA is caused by degeneration in the parts of the brain that are responsible for speech and language. PPA progressively worsens to the point where verbal communication by any means is very difficult. In this review, we synthesized clinical aspects, and classification of PPA, focusing on specific aspects like therapeutic strategies, for individuals with Primary Progressive Aphasia (PPA) focus on enhancing communication, supporting participation in daily life, and addressing psychosocial needs.
Keywords:Progressive primary aphasia; Therapeutic strategies for PPA
Introduction
It is estimated that 78 million people are projected to be affected by dementia by 2030 [1]. Current infrastructures to provide medical care to this population are frequently insufficient. The World Health Organization (WHO) Director General, dementia is a “looming… public health disaster, ‘a tidal wave’” [2,3]. Dementia is classified as young onset when it occurs before the age of 65 [4,5]. A review by Hendriks et al. [5] determined that approximately 370,000 people worldwide are diagnosed with young-onset dementia per year. The prevalence of young-onset dementia and far-reaching societal implications are significant. Young-onset dementias, ranging from Alzheimer’s disease to frontotemporal dementia to Parkinson’s disease, insidiously disrupt mechanisms of communicative competence [6,7] and therefore warrant speech and language intervention. Despite these differences, the functional impact of PPA encompasses all variants: A fundamental change and progressive loss to communicative ability.
Progressive Primary Aphasia
Definition
Progressive Primary Aphasia (APP) is a neurodegenerative pathology that stops an individual’s linguistic ability due to the selective involvement of the brain network assigned to language [8]. It is therefore a clinical syndrome characterized by speech and language disorders caused by the neurodegeneration of linguistic networks. The discussion of neurodegenerative aphasia remained in suspense until the 1970’s, when British researchers published several cases of progressive semantic loss, semantic dementia, and Mesulam and colleagues, first, introduced the term PPA [9]. In 1996, Grossman et al. also introduced the term progressive non-fluent aphasia, to describe patients with progressive loss of linguistic fluency. Over time it was noticed that many patients did not show the typical characteristics of semantic or non-fluent presentations; therefore, it was subsequently clarified that most of these subjects have a third subtype of APP, called the logopenic variant [10].
In 2011, an international group of experts introduced a common framework in which the APP was classified into three different variants, based on specific cognitive and neuroimaging characteristics: semantic variant (svPPA), non-fluent/agrammatical variant (nfvPPA) and logopenic variant (lvPPA) [11]. Recent clinicalpathological studies have shown that each variant is associated with different probabilities of neuropathological alterations and, rarely, with genetic mutations [12].
A person with APP has a language deficit with an insidious beginning and mainly shows: Difficulty in finding words and in using, understanding and constructing the sentence. Language disability is the most dominant characteristic that affects the patient’s daily activities. For the diagnosis of APP, a minimum follow-up of two years is necessary. It is important to emphasize that patients with aphasic symptoms associated with cognitive deficits cannot be classified as subjects with PPA, as the symptoms could be the result of the proper alteration of cognition. Similarly, if a person does not show aphasic symptoms, but only disorders of the Language, cannot be associated with APP [13,14]. However, the APPs share the pathologies with AD, Frontotemporal Lobar Degeneration (FTLD) with tauopathy and FTLD with TDP-43 protein precipitates [14].
Semantic variant progressive primary aphasia (svPPA)
Between 20 and 25% of patients diagnosed with FTD have svPPA [15]. Patients with svPPA have loss of knowledge of words and objects, and deficits are generally more severe for objects with low frequency of use and low familiarity. Symptoms include poor performance in comparison naming tests, individual word comprehension and object and face identification activities [12]. The semantic deficit begins with difficulties in the „more subtle” distinctions between things, such as the types of cars, moving on to the differentiation between the types of vehicles and finally to the loss of semantic knowledge of what vehicles are [16]. Subsequent studies have also shown that semantic loss also leads to superficial dyslexia: A disorder in which patients can read pseudowords but not words with exceptional spelling. Typical reading errors include the „regularization” errors of irregular words. Patients with svPPA often produce semantic paraphasias and tend to replace specific words, subordinate (in semantic hierarchies), with generalized terms, such as replacing „screwdriver” with „thing” and „panther” with „animal” [12].
For a decade, it was not clear whether semantic dementia and fluent APP were different entities. In 2004, Gorno-Tempini et al. [10] included semantic dementia as one of the three PPA variants and the term semantic variant PPA (svPPA) was subsequently adopted by the international working group. This new classification of svPPA includes the main classic characteristics of semantic dementia, such as anomie and word comprehension deficits. Supporting diagnostic characteristics include dyslexia or superficial dysgraphia, impaired knowledge of objects or faces, and relatively spared language production and repetition capabilities. Further reports have described deficits in different categories of objects and modes of stimuli, such as famous faces, voices, non-verbal ambient sounds, odors and taste stimuli, sometimes in relation to greater damage to the right anterior temporal lobe. In general, the distinctive feature of this disorder is the inability to identify the meaning of stimuli despite the preserved perception. As svPPA progresses, behavioral symptoms commonly occur including lack of empathy, personality changes, disinhibition, mental stiffness, and compulsive behaviors [12].
In addition to several clinical symptoms, the three main variants of PPA show distinct neuroimaging characteristics. Anterior temporal lobe damage in svPPA is identified as volumetric brain imaging atrophy (MRI) or as Fluorodeoxyglucose (FDGPET) hypometabolism. The damage is usually present in the left hemisphere, which is consistent with naming, word comprehension, and reading difficulties, but in about 30% of svPPA cases, atrophy is more severe in the right ATL. The latter will initially have difficulty identifying family members, recognizing facial expressions and showing behavioral abnormalities, such as loss of empathy, and repetitive and compulsive behaviors [12]. Thompson et al. also stated in 2003 that svPPA typically presents with left anterior temporal atrophy, but about 25% of cases present a more pronounced right-hand initial involvement than the left. In both cases, therefore, the contralateral anterior temporal lobe is affected by the progression of the disease. As the disease progresses, the multimodal loss of recognition of the person is also frequently observed [17].
Behavioral changes are initially present in patients with rightwing predominance, including lack of interpersonal commitment, lack of empathy, compulsive behaviors and increased rigidity expressed by strict schedules and limited food preference. As the disease progresses, the anatomical damage extends to the connected brain regions, and then to the posterior temporal lobes, contralaterally to the initially less involved ATL and anteriorly to the orbitofrontal regions. Semantic and language disorders consistently become more generalized, although flu islands may remain and may be useful for differential diagnosis later in the course of the disease. Typically, patients develop more obvious behavioral symptoms, while motor functions are usually spared up to the final stages, unless svPPA is associated with motor neuron disease [12]. With regard to the functional capacity of the person, the semantic deficit and the difficulties of voice and facial recognition, negatively affect the ability to engage socially, either through written correspondence, on the phone or face-to-face [18].
Although these ADLs are often initially compromised, the rate of progression is typically slower, in fact subjects with svPPA retain baseline ADL for much longer than patients with bvFTD and nfvPPA [15].
Driving ability can remain intact until more moderate stages of the disease, as well as language-mediated activities, despite the patient’s inability to name the elements or communicate their intentions [19]. The naming, single word comprehension, category fluency, and word-image correspondence tests are useful for isolating the main object loss deficit associated with svPPA [20]. In addition, svPPA patients make regularization errors when asked to read aloud or to try their hand at written dictation, where irregular words are read or written according to the rules of the sound of letters [21].
Non-fluent variant progressive primary aphasia (nfvPPA)
The key features of the nfvPPA variant are tiring language and/ or agrammatism, with a relative saving of semantic knowledge and understanding of a single word. Neuroimaging shows changes in the cortical-subcortical network anchored to the left posterior frontoinsular region, and the disorder is often associated with the FTLD spectrum of the tau subtype. The key clinical feature of nfvPPA is a motor speech disorder compatible with speech apraxia (AOS) and often with dysarthria [11]. Dysarthria is generally of a mixed type, with characteristics of both hypo phony and spasticity. Motor language planning difficulties cause speech sound errors and prosody distortions, sometimes referred to as phonetic and prosodic AOS [22]. Disturbances of motor language and phoneme selection also cause: substitutions, transpositions, insertions and deletions of sound.
The AOS is present especially when performing multiple repetitions of multisyllabic words that begin with consonant groups and that have various points of articulation, as in the words „artillery” or „catastrophe” (e.g. repeating „artillery” in quick succession for five times). Motor language (articulation) deficits are almost universally the most prominent feature of nfvPPA; when they appear to be isolated from other speech symptoms, the term Primary Progressive Word Apraxia (PPAOS) has been adopted. Agrammatism, the other important feature of nfvPPA, manifests itself mainly as a decrease in the average length of expression and a simplified grammar in oral and written speech. Omission of grammatical morphemes, incorrect use of inflection morphology and inaccurate order of words in spontaneous speech may also occur. NfvPPA patients produce fewer verbs than nouns and fewer functional words (e.g. prepositions, pronouns, conjunctions) than content words. Language tests show that syntactically complex sentences, such as passive sentences or embedded and objectrelated clauses, are particularly difficult for these subjects to produce and understand. When the deficits related to agrammatism are more evident than others, the term agrammatical PPA (AgPPA) is used.
Patients with AgPPA often show a prominent dysexecutive syndrome, such as working memory deficits, planning and setshifting [12]. Episodic memory and visuospatial abilities are relatively preserved in the nfvPPA, but may deteriorate over the course of the disease [15]. Series of longitudinal cases of patients with relatively isolated motor language deficits, or PPAOS, show that they eventually develop varying degrees of aphasic symptoms, suggesting that, in most cases, the PPAOS variant could be an early presentation within an nfvPPA spectrum, rather than a separate entity. Pathological studies seem to confirm this hypothesis, since both nfvPPA and PPAOS are mainly caused by the FTLD-tau disorder [23]. The acquisition of a language sample is the best clinical measure to highlight any agrammatisms and a reduced speed of speech. Aberrant behavior is not usually present in the initial presentation of nfvPPA, although it may occur with disease progression [15].
The site of the main brain damage in nfvPPA is in the pars opercular is of the left lower frontal gyrus (IFG) and premotor cortex, and atrophy can extend to the related cortical and subcortical regions, such as anterior insula, prefrontal regions, complex supplemental motor cortex, basal ganglia, and supramarginal gyrus. This network has been called the voice production network (SPN). The differential involvement of specific connections within this network probably explains the clinical variability within a non-fluent spectrum disorder: Pure motor (premotor cortex), agrammatical (prefrontal) or even dynamic (medial frontal) communication disorders. NfvPPA patients with early mutism have a cortical volume loss of the entire SPN network [12]. Similarly to patients with svPPA, speech, writing, and reading impairments occur early in patients with nfvPPA and significantly affect their ability to engage in interpersonal interactions. These disorders can be serious by the second year of the disease.
On the contrary, the basic ADLs remain relatively preserved for many years. As the disease progresses, patients can become mute and immobile, with more severe disabilities in understanding the sentence and following instructions consisting of several phases [15]. Patients with nfvPPA show fewer deficits in socio-emotional functioning than patients with svPPA, although recent research has shown that individuals with nfvPPA have selective deficits in interpreting emotions from vocal prosody [24,25]. Couto et al. [26] showed differential patterns of regional gray matter atrophy associated with cognitive-social deficits among patients with bvFTD and nfvPPA, suggesting that socio-emotional function deficits in nfvPPA may be a consequence of a basic dysfunction of facial and emotion recognition.
Primary progressive logopenic variant aphasia (lvPPA)
LvPPA represents the third variant of PPA; however, this syndrome is not classified as FTD given the association of this syndrome with Alzheimer’s disease [27,28]. The key clinical feature of the lvPPA is the difficulty in finding words, with pauses in spontaneous speech, considering the articulation and grammatical production relatively spared. The main cognitive deficit in lvPPA is a phonological and verbal disorder of short-term memory; these deficits manifest themselves with pauses to find words, with phonological paraphasias in the comparison denomination (especially for long words) and with difficulty in repeating long and unfamiliar sentences or strings of words or digits [12]. These cognitive deficits greatly affect speech disorders in lvPPA, since the immediate verbal recall is a fundamental factor in the repetition of sentences and statements and in the understanding of longer and grammatically complex sentences [15]. The diagnostic criteria for lvPPA therefore include deficits related to the recovery of a single word in spontaneous speech, the naming and repetition of sentences and statements [15].
The differential diagnosis between nfvPPA and lvPPA is more challenging, since both show varying degrees of impairment in speech fluency and sentence comprehension. In the nfvPPA, these deficits are due to deficits related to motor language and grammatical problems, while in the lvPPA they are probably secondary to short-term verbal auditory memory deficits. Unlike pauses in speech of subjects with nfvPPA, pauses of patients with lvPPA are largely caused by difficulties in finding words, so these patients, between pauses, present fluent language, with false departures and hesitations [12]. Sound errors in lvPPA are largely phonemic in nature (i.e. existing phonemes that are inserted, omitted or replaced in words) and non-phonetic (i.e. errors that produce non-existent distorted phonetics). Individuals with lvPPA struggle in comparison naming tasks, although to a lesser extent than individuals with svPPA. Other deficits are related to dyscalculia and ideomotor apraxia [12].
The left Lower Parietal Lobe (LPL), the left posterior temporal lobe and the left Temporoparietal Junction (TPJ) are constantly involved in lvPPA, as demonstrated by volumetric MRI and FDGPET analysis. Naming difficulties in lvPPA are related to the atrophy of the left middle time turn, while repetition deficits are related to damage to the temporoparietal junction. A part of these networks is included in the circuit involved in Alzheimer’s disease, which supports clinical-pathological and biomarker studies that show that this syndrome is an atypical variant of Alzheimer’s disease. In fact, Cerebrospinal Fluid (CSF) analysis shows a consistent pattern with AD disease. PET molecular studies have also confirmed the presence of amyloid deposit in about 90% of patients with lvPPA [12].
LvPPA often progresses to global aphasia, with episodic memory impairment, disecutive and visuospatial dysfunction, similar to the clinical picture of the patient with early-onset Alzheimer’s disease. Anxiety, irritability, agitation and depression have also been reported, while frank disinhibition and lack of empathy are rare [12].
Language tests to evaluate the APP
The nfvPPA is determined using activities focused on grammar, sentence comprehension and linguistic articulation. Activities such as describing images, storytelling, and producing sentences help measure grammatical structure, accuracy, speed of speech, and the type of specific errors in word selection and articulation. The repetition of multisyllabic words and spontaneous speech activities are used in the evaluation of the type of speech sound errors, the factors that influence the articulation and the presence of speech apraxia. Matching images, following directions and answering yes/ no questions, help evaluate the factors that affect grammatical complexity and understanding.
Similarly, svPPA is determined using activities focused on language functions such as naming, object knowledge, understanding individual words, spelling, and reading. Activities such as retrieving words from images, sounds, smells, etc. are helpful in understanding the error rate, the delay in naming, and the factors that affect naming. The combination of words with images, definitions and synonyms, help to measure the factors that influence understanding. The matching of images with images and sounds, the matching of gesture to the object, and word association activities, are useful for measuring factors that affect knowledge of the object such as familiarity and semantic category. The lvPPA is determined using activities based on repetition, naming and spelling. Tasks such as the repetition of words, not words, sentences and statements, the retrieval of words and reading, are useful in measuring the factors that affect the articulation and type of phonemic errors [11].
Diagnostic-linguistic criteria of the three variants of APP
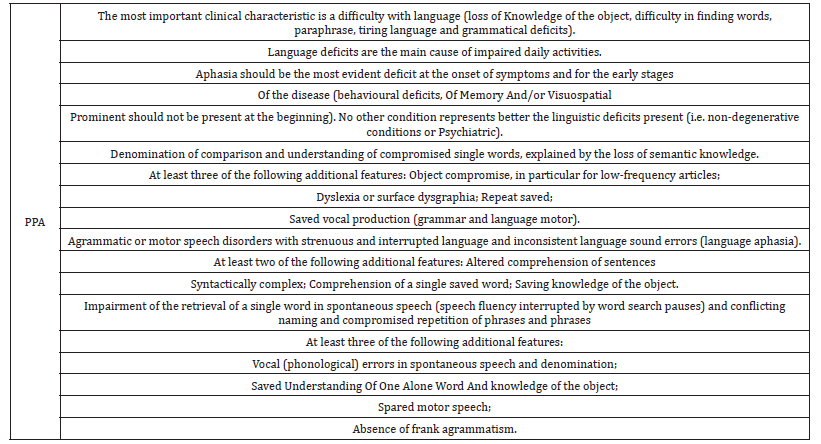
Table 1 shows a summary of the main criteria elaborated by the international consortium in 2011 for the diagnosis of APP and the three respective variants [11].
Table 1:Summary of international consensus criteria for clinical diagnosis and classification of progressive primary aphasia.
Therapeutic strategies for PPA
There is growing evidence to support the effectiveness of speech-focused rehabilitation therapy in APPs, with or without the addition of non-invasive brain stimulation [12]. This intervention is important because PP and speech disorder in other NDDs are the main stressors for patients, caregivers and therapists [8]. In the nfvPPA, motor language disorders and agrammatism showed positive effects in subjects undergoing structured oral reading tasks training and video-implemented script training for aphasia therapy. Lexical recovery treatments are one of the most widely explored non-pharmacological therapies in these cases. To maximize its therapeutic effects, it is essential to focus on spared language skills, such as phonological and autobiographical memory processes in svPPA and semantic memory capabilities in lvPPA. A recent study shows that naming intensive care is associated with increased bilateral activation at post-treatment functional magnetic resonance imaging [12].
The structured oral reading strategy is used for word retrieval, for better production of multisyllabic words and for grammatical processing in nfvPPA. The inability to retrieve words, especially verbs, seriously alters the wording of the sentence. This is overcome through structured word learning. This results in improved sentence production and is limited to the strategy used. In addition, the accuracy of the sentences used as training, helps in the production of sentences with similar syntactic structure (this process is known as generalization). It has been shown that the combination of transcranial Direct Current Stimulation (tDCS) with linguistic production activities, ensure better treatment for PPA [8], tDCS is a mechanism that alters the membrane potentials of the neurons of a target brain area and helps in the activation of the neural circuit. Neural activation increased by tDCS enables the brain to recruit undamaged areas to take on the functions of damaged ones. These changes are significantly associated with better language performance during reading and naming tasks.
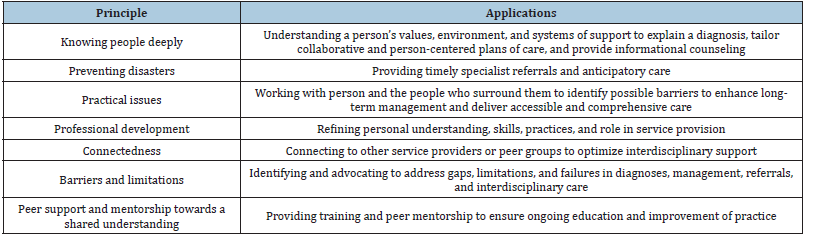
Lasting improvements have been recorded in certain language performance, especially in verbal word learning, thus confirming memory formation after several sessions. So, while memory decline is one of the main causes of speech dysfunction, tDCS offers a promising intervention in the treatment of these NDDs, decreasing the degree of decline in neurodegeneration [8]. PPA served as a model to demonstrate that targeted cognitive therapy can be useful for improving and delaying the progression of cognitive symptoms in neurodegenerative disorders [12]. In Table 2 (the best practice principles for speech -language therapists/pathologists).
Table 2:A description of the best practice principles for speech-language therapists/pathologists working with primary progressive aphasia (Ruggero et al.) [34,35,38].
Conclusion
A review by Abdulrahim Saleh Alrasheed and colleagues investigated the safety and efficacy of various therapeutic interventions for Primary Progressive Aphasia (PPA). The review demonstrate that several therapeutic interventions are effective in improving language function in individuals with PPA. Speechlanguage therapy, when combined tDCS, has been associated with consistent gains in naming ability, speech fluency, and lexical retrieval. Group-based therapy approaches have also shown beneficial effects in enhancing both communication skills and social interaction. The NIBS, when used alone, has shown limited effectiveness, and pharmacological interventions have not substantial improvements in language function. These findings support the adoption of personalized, multimodal therapeutic strategies that integrate behavioral and neuro-modulatory interventions to optimize communication outcomes in individuals with PPA [29]. The capacity to communicate is a feature of the human experience. A behavioral approach to the care of people living with PPA (PwPPA) is paramount to enhance, maintain, or compensate for communication loss. There is no curative treatment available for this condition [30].
Speech and language therapy is the primary intervention that can slow down the behavioral impact of symptoms, which impacts positive, on quality of life. Such intervention has been shown to maintain communication abilities for longer through both compensatory and restitutive approaches [31,32]. Speech and language therapy is imperative to optimize possible improvements, maintenance, and compensation for decline in linguistic capacity faced by PwPPA [33,34].
Speech-language therapists/pathologists (SLT/Ps) are recognized as central specialists in the care of PwPPA with roles in the diagnosis and coordinated care of the condition [30,34-38]. Kate Swaffer, founder of Dementia Alliance International (DAI), and person living with the semantic variant of PPA, has spoken publicly about the pressing need for SLT/Ps to be consistently integrated in the care of those living with the condition. “People with dementia who will almost all have language and speech changes, need proactive speech pathology after diagnosis, not just a speech pathologist to attend when we can no longer swallow well nearer to the end of life… this is how I have managed to still speak as well as I do.” [39].
Behavioral interventions such as language and speech therapy, however, are outside the clinical context and are nonpharmacological in nature. Both behavioral and clinical interventions help in language recovery. It’s necessary a multidisciplinary approach of the perspectives of general practitioners, neurologists, neuropsychologists, and other team members in the care of PwPPA, for identify and hopefully reduce possible obstacles in the comprehensive care necessitated by this condition.
References
- ADI - Dementia statistics.
- Prince M, Wimo A, Guerchet M, Ali G, Wu Y, et al. (2015) World Alzheimer Report 2015. The Global Impact of Dementia. An analysis of prevalence, incidence, cost and trends. pp. 1-24.
- Zimmerman R (2015) Dementia as a global public health ‘Tidal wave’. WBUR News.
- Koopmans R, Rosness T (2014) Young onset dementia – what does the name imply? International Psychogeriatrics 26(12): 1931-1933.
- Hendriks S, Peetoom K, Bakker C, Koopmans R, Flier W, et al. (2023) Global incidence of young-onset dementia: A systematic review and meta analysis. Alzheimer’s Dementia 19(3): 831-843.
- Vuorinen E, Laine M, Rinne J (2000) Common pattern of language impairment in vascular dementia and in Alzheimer disease. Alzheimer Disease and Associated Disorders 14(2): 81-86.
- Kimbarow ML, Wallace SE (2023) Cognitive communication disorders. (4th edn), Plural Publishing, USA, pp. 1-550.
- Rahul DR, Ponniah J (2019) Language impariment in primary progressive aphasia and other neurodegenerative diseases. Journal of Genetics 98.
- Mesulam MM (1987) Primary progressive aphasia–differentiation from Alzheimer’s disease. Ann Neurol 22(4): 533-534.
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, et al. (2004) Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55(3): 335-346.
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, et al. (2011) Classification of primary progressive aphasia and its variants. Neurology 76(11):1006-1014.
- Boon LT, Gorno-Tempini ML (2019) Primary progressive aphasia: A model for neurodegenerative disease. Curr Opin Neurol 32(2): 255-265.
- Mesulam MM (2001) Primary progressive aphasia. Ann Neurol 49(4): 425-432.
- Mesulam MM (2013) Primary progressive aphasia and the language network: The Houston Merritt Lecture. Neurology 81(5): 456-462.
- Bott NT, Radke A, Stephens ML, Kramer JH (2014) Frontotemporal dementia: Diagnosis, deficits and management. Neurodegener Dis Manag 4(6): 439-454.
- Hodges JR, Patterson K, Oxbury S, Funnell E (1992) Semantic dementia. Progressive fluent aphasia with temporal lobe atrophy. Brain 115(Pt 6): 1783-1806.
- Snowden JS, Thompson JC, Neary D (2004) Knowledge of famous faces and names in semantic dementia. Brain 127(Pt 4): 860-872.
- Rabinovici GD, Miller BL (2010) Frontotemporal lobar degeneration: Epidemiology, pathophysiology, diagnosis and management. CNS Drugs 24(5): 375-398.
- O'connor C, Ahmed S, Mioshi E (2014) Functional disability in primary progressive aphasia. Aphasiology 28(8-9): 1131-1149.
- Perry RJ, Hodges JR (2000) Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer's disease. Neurology 54(12): 2277-2284.
- Shim H, Hurley RS, Rogalski E, Mesulam M (2012) Anatomic, clinical, and neuropsychological correlates of spelling errors in primary progressive aphasia. Neuropsychologia 50(8): 1929-1935.
- Grossman M (2012) The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol 11(6): 545-555.
- Santos MA, Mandelli ML, Binney RJ, Ogar J, Wilson SM, et al. (2016) Features of patients with nonfluent/agrammatic primary progressive aphasia with underlying progressive supranuclear palsy pathology or corticobasal degeneration. JAMA Neurol 73(6): 733-742.
- Rohrer JD, Sauter D, Scott S, Rossor MN, Warren JD (2012) Receptive prosody in nonfluent primary progressive aphasias. Cortex 48(3): 308-316.
- Shany-Ur T, Rankin KP (2011) Personality and social cognition in neurodegenerative disease. Curr Opin Neurol 24(6): 550-555.
- Couto B, Manes F, Montanes P, Matallana D, Reyes P, et al. (2013) Structural neuroimaging of social cognition in progressive non-fluent aphasia and behavioral variant of frontotemporal dementia. Front Hum Neurosci 7: 467.
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S (2012) Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain 135(Pt 5):1537-1553.
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, et al. (2014) Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain 137(Pt4): 1176-1192.
- Alrasheed AS, Alshamrani RA, Al Ameer AA, Alkahtani RM, AlMohish NM, et al. (2025) Safety and efficacy of different therapeutic interventions for primary progressive aphasia: A systematic review. J Clin Med 14(9): 3063.
- Belder CRS, Marshall CR, Jiang J, Mazzeo S, Chokesuwattanaskul A, et al. (2024) Primary progressive aphasia: Six questions in search of an answer. J Neurol 271: 1028-1046.
- Volkmer A, Spector A, Warren JD, Beeke S (2020) Speech and language therapy for primary progressive aphasia: Referral patterns and barriers to service provision across the UK. Dementia 19(5): 1349-1363.
- Wauters LD, Karen Croot, Dial HR, Duffy JR, Grasso SM, et al. (2023) Behavioral treatment for speech and language in primary progressive aphasia and primary progressive apraxia of speech: A systematic review. Neuropsychol Rev 34(3): 882-923.
- Cadório I, Lousada M, Martins P, Figueiredo D (2017) Generalization and maintenance of treatment gains in Primary Progressive Aphasia (PPA): A systematic review. International Journal of Language & Communication Disorders 52: 543-560.
- Volkmer A, Bruns C, Zimmerer V, Varley R, Beeke S (2023) Giving voice to people with dementia and their carers: The impact of communication difficulties on everyday conversations. International Journal of Qualitative Methods 22: 1-15.
- Gallée J, Volkmer A (2023) Role of the speech-language therapist/pathologist in primary progressive aphasia. Neurol Clin Pract 13(4): e200178.
- Gallée J (2023) A roadmap to enhance care for people living with primary progressive aphasia: What can be done now? Perspect ASHA SIGs 8(5): 847-862.
- Lo KC, Bricker-Katz G, Ballard K, Piguet O (2022) The affective, behavioural, and cognitive reactions to a diagnosis of primary progressive aphasia: A qualitative descriptive study. Dementia (London) 21(8): 2476-2498.
- Ho T, Whitworth A, Hersh D, Cartwright J (2023) “They are dealing with people’s lives…”: Diagnostic and post-diagnostic healthcare experiences in primary progressive aphasia. International Journal of Speech-Language Pathology 25(3): 449-461.
- Swaffer K (2022) Hello, my name is Kate. Dementia Alliance International.
© 2025 Letteria Tomasello. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






