- Submissions

Full Text
Examines in Physical Medicine and Rehabilitation: Open Access
Autologous Stromal Vascular Fraction and Microfragmented Adipose Tissue for Musculoskeletal Disorders: A Narrative Review
Katz Nathan1*, Pancholi Nishit1, Katz Michelle2 and Sheinkop Mitchell1,3
1Jointechlabs Inc., USA
2Tulane University, School of Science and Engineering, USA
3Cellular Orthopedics, USA
*Corresponding author:Katz Nathan, Jointechlabs Inc., 421 Lithia Pinecrest Road, Brandon, FL 33511 USA
Submission: April 02, 2024;Published: July 09, 2024

ISSN 2637-7934 Volume5 Issue1
Abstract
Recently, Adipose-Derived Stem Cells (ADSCs) have been identified as potential therapeutic solutions for treating musculoskeletal disorders. However, the use of culture expanded ADSCs and ADSCs obtained by traditional enzymatic digestions which is otherwise known as Stromal Vascular Fraction (SVF) is strictly regulated by complicated legislation. Thus, several attempts have been made to isolate ADSCs through mechanical/non-enzymatic methods and without expansion at the point of care, leading to the development of minimally manipulated products-mechanically isolated SVF and Microfragmented Adipose Tissue (MFAT). Notably, although several studies documented the safety and efficacy of ADSCs for treating musculoskeletal diseases, the majority of them focused on culture expanded ADSCs rather than uncultured ADSCs (MFAT or SVF). To date, randomized controlled trials that have compared uncultured ADSCs with orthobiologics (hyaluronic acid, autologous blood derivates, and bone marrow concentrate) are limited. This review article aimed to provide a comprehensive review of randomized controlled trials that evaluated the safety and efficacy of uncultured ADSCs for treating musculoskeletal disorders while directing the reader’s attention towards heterogeneity in MFAT and SVF processing methods, characterization of stem cells, injectable dose, and techniques, and inconsistencies in reported clinical and imaging outcomes.
Keywords:Achilles tendinopathy; Microfragmented adipose tissue; Osteoarthritis; Stromal vascular fraction; Temporomandibular joint disease; Uncultured adipose-derived stem cells
Introduction
Musculoskeletal disorder is defined as any discomfort to irreversible and disabling injury that affects the motor organs, muscles, tendons, bones, cartilage, ligaments, and the nerves. According to the recent Global Burden of Disease (GBD) study, approximately 1.71 billion people are suffering from musculoskeletal conditions [1]. As these disorders impact one’s life course, they are of great clinical significance. Despite their tremendous impact on health, initial treatment approaches are palliative. Recently, orthobiologics have been extensively investigated for treating various musculoskeletal disorders. Orthobiologics are biological products derived from naturally found substances in the human body that optimize the local biological environment to facilitate the repair of the tissues that otherwise have limited inherent healing capacities such as cartilage, muscle, tendon, ligament, and meniscus. Orthobiologics include Hyaluronic Acid (HA), autologous blood derivates, bone marrow concentrate, micronized adipose tissue and adult stem cells.
Research has focused on the clinical application of stem cells for various disease that include embryonic stem cells, induced pluripotent stem cells, and adult stem cells such as Mesenchymal Stromal Cells (MSCs) [2]. Ethical issues, risk of developing teratoma, and immune responses to implantation of embryonic stem cells limit clinical applications of stem cells [3]. Although implantation of induced pluripotent stem cells is devoid of such concerns, cell preparation is labour-intensive and technically challenging [2]. Contrastingly, MSCs can be derived and isolated from diverse tissues such as bone marrow, adipose tissue, synovium, endometrium, peripheral blood, and from allogenic sources such as placenta, umbilical cord, and amniotic fluid [4]. Notably, the ideal source of stem cells is yet to be identified. However, Bone-Marrow-Derived MSCs (BMMSCs) and Adipose-Derived Stem Cells (ADSCs) have been extensively studied and are considered emerging areas of research for various orthopedic applications.
The ADSCs have several advantages over BM-MSCs:
a. In vitro studies demonstrate that ADSCs maintain their
phenotype for a longer period and exhibit higher proliferation
rates.
b. ADSCs can be easily harvested in sufficient quantities from
subcutaneous adipose tissues (the abdomen, thigh, or buttock)
using hand-held syringes or machine-generated vacuum
pressure and a liposuction cannula; further, the procedure is
less invasive and less painful than the one performed to obtain
BM-MSCs.
c. There is great variability in MSCs observed in bone
marrow aspirate and lipoaspirate. So, one ml sample of the
bone marrow aspirate yields approximately 6×106 nucleated
cells, of which approximately 0.01% cells are true BMMSCs.
Whereas approximately 2×106 nucleated cells are present in
one gram of lipoaspirate and nearly 10% of these are ADSCs
[5-7].
Considering these advantages, ADSCs are the most attractive source of MSCs over BMMSCs for regenerative medicine. Yet, it should be noted that the clinical effect is not necessarily attributed to the presence of MSC only but to the multiple cells present in adipose and bone marrow stroma. Due to differences in the paracrine activities of ADSC and BM stromal cell concentrate (BMAC), the clinical effect may be attributed to the different stimulators and as such address disease modification from different mechanisms of action.
Several pre-clinical and clinical studies have documented the efficacy of culture expanded ADSCs for treating degenerative orthopaedic disorders. However, clinical applications of cultured expanded ADSCs are not practically feasible. Further, they lack supportive cells that facilitate regeneration and repair. In contrast, ADSCs derived from enzymatic digestions of the lipoaspirate are referred to as the Stromal Vascular Fraction (SVF). The SVF, which is derived from enzymatic digestion is called cellular SVF and is a heterogeneous and synergistic mixture of cells that includes endothelial cells, monocytes, lymphocytes, myeloid cells, pericytes, pre-adipocytes, smooth muscle cells, and MSCs. Thus, SVF has supportive cells that modulate the microenvironment through paracrine effects to facilitate repair and regeneration. In addition, they can be easily acquired without needing any cell separation/ culturing conditions.
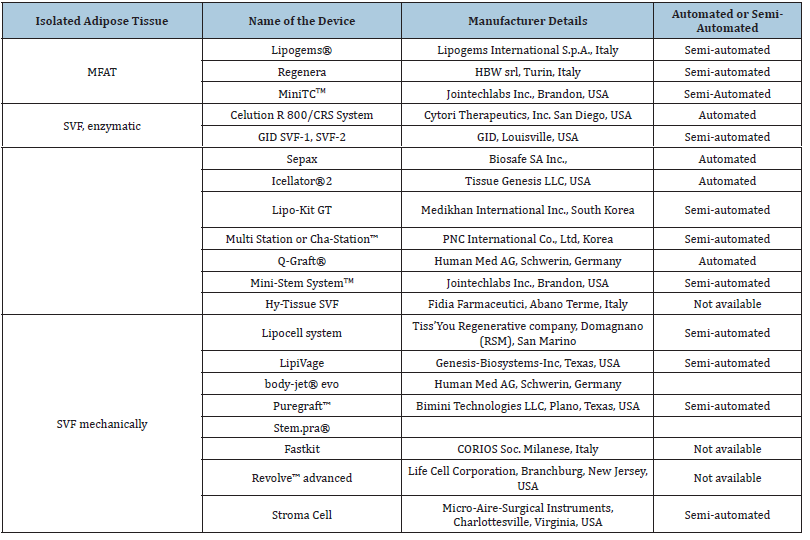
However, the United States Food and Drug Administration (US FDA) and European Medicine Agency (EMA) yet consider enzymatic digestions to isolate SVF under “substantial manipulations” as this processing alters original yet relevant characteristics of the adipose tissue which affects its ability to provide cushioning and support. Further, enzymatic digestion of tissue also requires the use of xenogeneic substances that is discordant with the European Good Manufacturing Practice (eGMP) Guidelines (Regulation (EC) No. 1394/2007 of the European Parliament and the European Council). Hence, the clinical application of autologous use of SVF is imparted with strict regulations and requires an FDA-approved Biologic License application. This regulatory status quo was challenged in courts yet remains an official at the time of writing current manuscript. Consequently, non-enzymatic methods to isolate SVF have been introduced to exempt regulatory requirements, and they are based on centrifugal force, pressure, filtration, and washing to isolate ADSCs from the lipoaspirate. The end product received after non-enzymatic processing is a mixture that contains cellular debris, blood cells, and extracellular matrix fragments and is collectively called “tissue SVF”. Within recent decade a concentration method for ADSCs was introduced which was a combination of washing and passing lipoaspirate through a size-reduction filter that allows the collection of small clusters of fat (around 300μm to 800μm), and the resultant product was called Microfragmented Adipose Tissue (MFAT). Several semi-automated and automated devices (Table 1) have been developed to isolate SVF from a relatively smaller volume of lipoaspirate with minimal training, and following protocols. They also avoid the risk of viral/bacterial infection and inconveniences associated with cell culture and multiple-step techniques.
Table 1:Commercially available devices for harvesting and processing adipose tissue to isolate SVF or MFAT adopted from Oberbauer et al. [26] and Mazini et al. [27].
A literature review identified several systematic reviews and meta-analyses that evaluated the efficacy of ADSCs for treating musculoskeletal disorders, mostly OA [8-11]. Nevertheless, they also include heterogeneous studies in terms of autologous or allogenic ADSCs, adjuvant treatments, delivery methods, and level of evidence of included studies which may probably explain the inconsistent efficacy of ADSCs for treating musculoskeletal disorders. Thus, this review summarizes the efficacy and safety of uncultured ADSCs (MFAT/ SVF) in treating various musculoskeletal disorders reported by Randomized Controlled Trials (RCTs).
Methods
A literature search on PubMed was performed in June, 2023 using the following search terms: „adipose tissue-derived mesenchymal stem cell*”, „adipose tissue-derived stem cell*”, „adipose derived stem cell*”, „adipose tissue-derived stem cell*”, „adipose tissue-derived stromal cell*”, „adipose tissue-derived stromal cell*”, „Stromal vascular fraction*”, „adipose tissuederived stromal vascular fraction*”, microfat, microfragmented, „micro-fragmented”, nanofat and orthopaedic*, orthopaedic*, arthritis, osteoarthritis, „rheumatoid arthritis”, „joint disease*”, „joint disorder*”, „joint pain”, „joint effusion”, „joint arthrosis”, „rheumatoid nodule*”, „cartilage regeneration”, „cartilage repair”, tendinitis, tendinopathy, „knee articular cartilage injury”, „cartilage injury”, „cartilage defect*”, „chondropathy”, „non-union fracture*”, „avascular necrosis”, osteonecrosis, osteoporosis, osteoarthrosis, „osteoarthrosis deformans”, „osteoporotic fracture*”, „rheumatoid nodule”, „systematic lupus erythematosus”, „knee injury”, „knee injuries”, „genu verum”. This search yielded 486 results. Any articles not written in English were excluded. The titles and abstracts of the remaining articles were reviewed by initials of author(s) to identify all studies which utilized implantation of uncultured ADSCs to treat bone, cartilage, tendon, ligament, or meniscal injury. Preclinical studies or articles associated with a surgical procedure or involved in culture expansion of cells were also excluded. Only RCTs that compared uncultured ADSCs with currently available treatment/ placebo were included. The reference list of systematic reviews and the included articles were also screened against the aforementioned criteria. In total, 12 RCTs were included in this review (Tables 2&3).
Table 2:Summary of randomized controlled trials that assessed efficacy of SVF/MFAT for treatment of musculoskeletal.
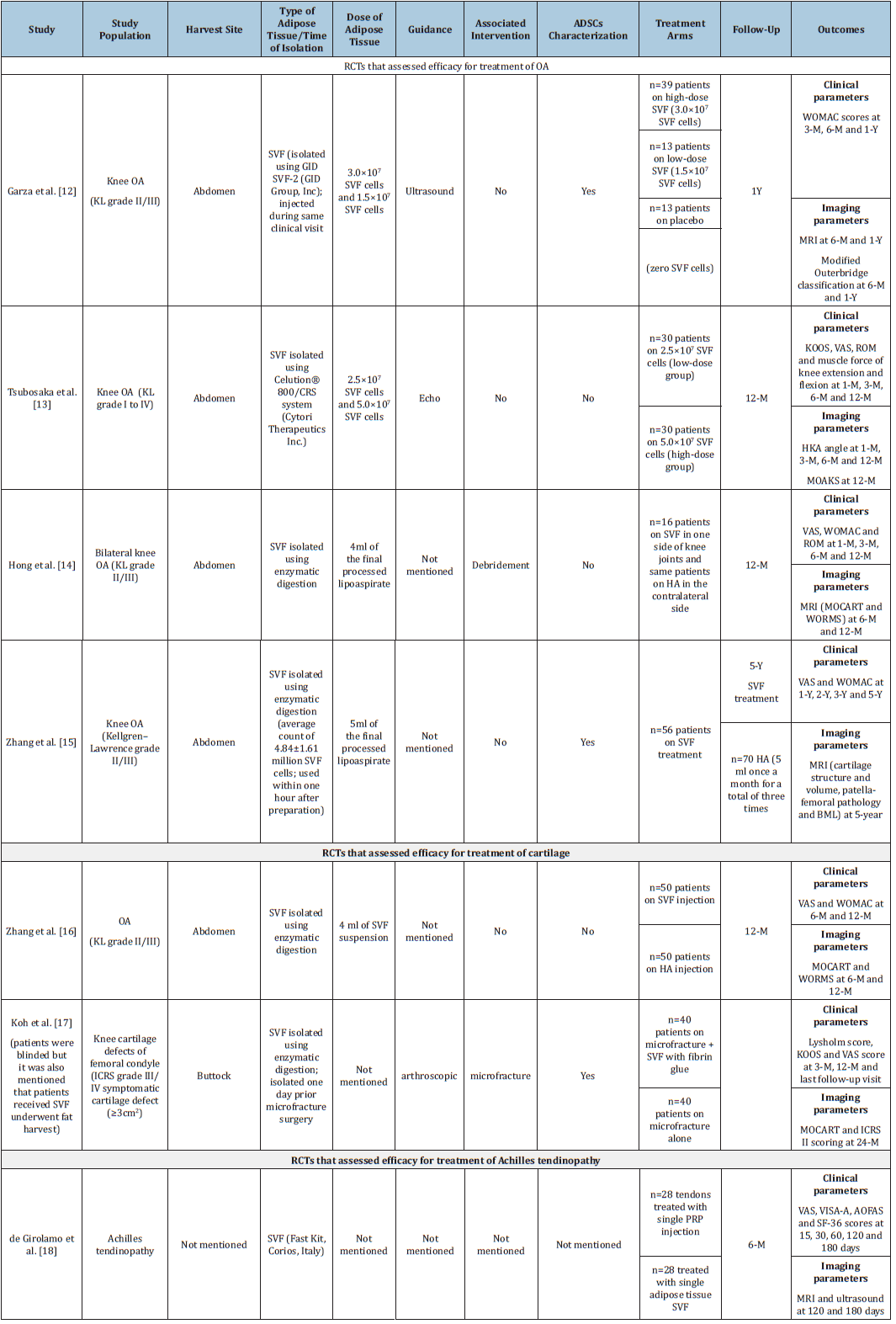
Note: ADSC: Adipose-Derived Stem Cells; AOFAS: The American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Score; BML: Bone Marrow Lesion; HA: Hyaluronic Acid; ICRS: International Cartilage Repair Society; KOOS: Knee Injury and Osteoarthritis Outcome Score; KL: Kellgren-Lawrence; MRI: Magnetic Resonance Imaging; MOCART: Magnetic Resonance Observation of Cartilage Repair Tissue; ROM: Range of Motion; SF-36: Short Form-36; SVF: Stromal Vascular Fraction; VAS: Visual Analogue Score; VISA-A: Victorian Institute of Sports Assessment; WORMS: Whole-Organ Magnetic Resonance Imaging; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3:Summary of randomised controlled trials that assessed efficacy of MFAT/MF for treatment of musculoskeletal disorders.
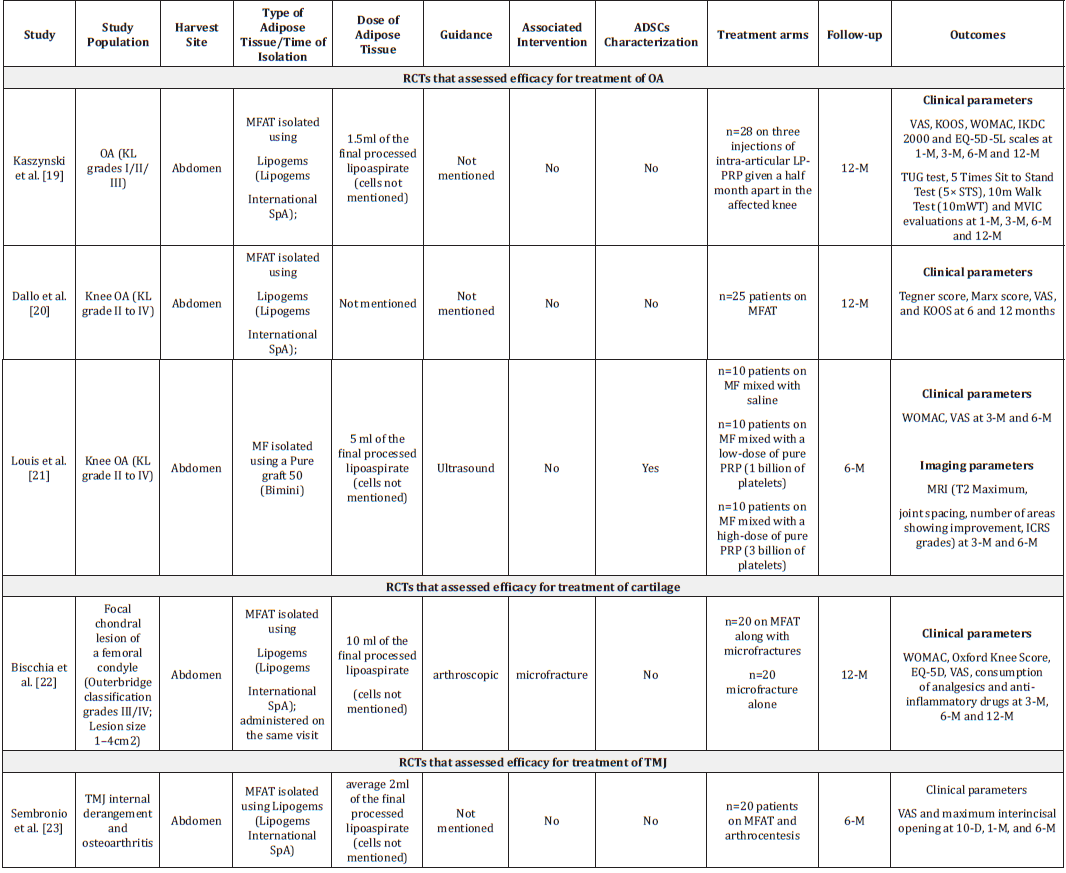
Note: ADSC: Adipose-Derived Stem Cells; EQ-5D: EuroQol- 5 Dimension; HA: Hyaluronic Acid; ICRS: International Cartilage Repair Society; KOOS: Knee Injury and Osteoarthritis Outcome Score; KL: Kellgren-Lawrence; MF: Microfat; MFAT: Microfragmented Adipose Tissue; MRI: Magnetic Resonance Imaging; MOCART: Magnetic Resonance Observation of Cartilage Repair Tissue; ROM: Range of Motion; TMJ: Temporomandibular Joint; TUG: Timed Up & Go test; VAS: Visual Analogue Score; WORMS: Whole-Organ Magnetic Resonance Imaging; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Result
Efficacy and safety of SVF for the treatment of musculoskeletal disorders
Of 12 RCTs that assessed safety and efficacy of uncultured ADSCs for treating various musculoskeletal conditions, 4, 2 and 1 studies reported efficacy and safety of SVF for treatment of OA, cartilage and Achilles tendinopathy, respectively.
Treatment of OA: To investigate the efficacy of intra-articular SVF compared to placebo, Garza et al [12]. carried out a prospective multicenter, double-blinded randomized placebo-controlled trial. In this study, 39 patients with knee OA (Kellgren-Lawrence grade II/III) were randomly allocated (1:1:1) to receive either high-dose SVF (3.0×107 SVF cells) or low-dose SVF (1.5×107 SVF cells) or placebo (no SVF cells). The SVF was isolated using GID SVF-2 tissueprocessing device; isolated SVF was administered to the patient during a single visit only. The WOMAC scores at 6-month and 1-year follow-ups demonstrated significantly greater clinical benefits with SVF treatments compared to placebo. Further, the doseresponse curve and effect size assessments showed significantly greater therapeutic efficacy with high-dose SVF than low-dose SVF. It should be noted that Magnetic Resonance Imaging (MRI) evaluation at 6-month follow-up revealed no changes in cartilage loss or severity assessment using Outerbridge classification in all three treatment groups. Reported Adverse Events (AEs) only included knee swelling.
In another study, 60 patients with OA (Kellgren-Lawrence grade I to IV) were randomized to receive either intra-articular injection of 2.5×107 SVF cells (low-dose group; n=30) or an intra-articular injection of 5.0×107 SVF cells (high-dose group; n=30 patients) [13]. The SVF cells were extracted using the Celution® 800/CRS system (Cytori Therapeutics Inc., San Diego, CA, USA) and their cell count, and viability calculation was performed using NC-100™ NucleoCounter® Automated Cell Counting System (ChemoMetec, Allerod, Denmark). Clinical evaluation at 6- and 12-month followups showed improvement in extension angle, flexion muscle force, VAS, and pain subscale score of KOOS from baseline. Notably, knee injury and osteoarthritis outcome score (KOOS) total scores, pain subscale, symptoms subscale, activity of life subscale, and quality of life scores of KOOS were better in the high-dose group than the low-dose group. However, there was significant improvement in imaging evaluation (as measured by heap-knee-ankle angle, Bone Marrow Lesion [BML], cartilage defect improvement rates, Hoffa’s synovitis improvement rates, and effusion synovitis improvement rates) at 12 months from baseline in both groups. However, there was no significant difference in imaging evaluations between both groups. Few incidences of mild AEs such as swelling and pain in both treatment groups were seen but disappeared within three days, and the treatments were well-tolerated.
Hong et al. [14] performed a double-blind, randomized selfcontrolled trial to assess clinical and radiological efficacy of autologous adipose-derived SVF compared to HA in 8 patients with bilateral knee OA (Kellgren-Lawrence grade II/III) [14]. Each patient was treated with either autologous adipose-derived SVF treatment (single intra-articular injection of 4ml of SVF suspension; n=16 knees) on one knee joint and a single dose of HA (single intraarticular injection of 40mg HA; n=16 knees) on the contralateral side. Those knees that received adipose-derived SVF showed significant improvement in the mean Visual Analogue Scale (VAS), WOMAC pain and stiffness scores, and Range of Motion (ROM) throughout the study period. On the other side, knees treated with HA initially showed significant improvement as evident by improved VAS score (at 1- and 3-month) and ROM (at 1-month). However, these parameters worsened later (at 6 months and 12 months). There was worsening of WOMAC pain and stiffness subscores for knees treated with HA. Clinical efficacy findings are supported by radiological evaluation as there was significant improvement in Whole-Organ Magnetic Resonance Imaging (WORMS) score from baseline to 6- and 12-month for knees treated with adiposederived SVF treatment but deterioration in the scores for knees treated with HA. Radiographical evaluation of articular cartilage defects through the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score showed significant improvement in the cartilage for knees treated with adipose-derived SVF treatment at 6- and 12-month but deterioration was seen in the cartilage for HA-treated knees. However, cartilage repair was not confirmed with second-look arthroscopy or biopsy. Further, this study did not provide any insight on the actual association between SVF cell density, cell viability, and outcomes. It reported few AEs related to knee surgery (pain and swelling) and adipose harvest (muscle soreness after strenuous exercise) but were managed by Celebrex. However, none of the patients experienced major AEs related to the knee surgery (including infection, allergy, and poor wound healing) and adipose harvest (including deformity and severe ecchymosis).
While all the aforementioned trials reported short-term outcomes for treating knee OA, only one assessor-blinded RCT reported clinical outcomes of SVF treatment at 5-year follow-up [15]. A total of 126 patients with knee OA (Kellgren–Lawrence grade II/III) were randomly allocated to receive SVF-treatment (average count: 4.84±1.61 million viable SVF cells; injected within one hour after preparation once in a month for three times; n=56) or HA (5ml once a month for three times; n=70). Clinical evaluation demonstrated significant improvement in the VAS scores from baseline (at years 1, 2, 3 and 5) and the WOMAC scores (at years 1, 2, and 3) in patient’s received SVF treatment. On the contrary, VAS and WOMAC scores in the HA group did not differ at different time points from baseline. Comparison between both treatment groups at different time points (after eliminating cross-over effects) demonstrated significant improvement regarding VAS and WOMAC scores at different time points after treatment with SVF compared to those treated with HA. The MRI demonstrated that patients treated with SVF were more likely to reduce or change the grade of full-thickness cartilage defects, and less likely experience disease progression as compared to their counterparts. However, there was no change in BML size, severity, patella-femoral pathology or mechanical axis from baseline to 5 years and no difference between both treatments. This study also identified BML severity score, BMI and treatment as independent risk factors for clinical failure, defined as surgeries related to knee OA (total knee arthroplasty, unicondylar knee arthroplasty and debridement under arthroscopy) or clinical scores exceeding the patient acceptable symptom state (VAS >3.23 or WOMAC function score >31). Remarkably, SVF treatment reduced the risk for clinical failure by 2.602 times. The 5-year responsive rate of the SVF group was significantly better than HA and exceeded 60% and indicated that patients treated with SVF were less likely to experience clinical failure in 5 years.
Treatment of cartilage:The prospective double-blinded randomized clinical trial study carried out by Zhang et al. [16] evaluated efficacy of SVF versus HA in cartilage regeneration by establishing a cartilage model based on three-dimensional fatsuppressed spoiled gradient recalled echo (3D-FS-SPGR) sequence [16]. Patients (N=100) with symptomatic OA were recruited and equally randomized to receive either SVF injection (n=50) or HA injection (n=50) and were graded II-III according to the Kellgren- Lawrence (K-L) criteria. Each patient underwent the 3D-FS-SPGR sequence to establish a cartilage model at baseline, 6 months, and 12 months, respectively. In patients given SVF injection, the thickness and volume of cartilage defect decreased by in medial femoral condyle and in medial tibia condyle. SVF-treated knees showed significant improvement in clinical and radiographic scores at 12 months to those given HA. Nevertheless, these scores of the HA treated patients became worse at 12-month follow-up visit. Thus, intra-articular SVF injection markedly improved clinical symptoms (relieves pain and improves function) and without adverse events, thereby repairing the damaged articular cartilage through cartilage regeneration is a promising minimally invasive therapy.
A prospective randomized, non-blinded trial that compared clinical and radiologic efficacy of SVF with fibrin glue and Microfracture (MFX) versus MFX alone in patients with symptomatic knee cartilage defects (International Cartilage Repair Society grade III/IV symptomatic cartilage defect (≥3cm2) on the femoral condyle in a stable, well-aligned knee [17]. Patients were randomized into groups receiving MFX and SVF (n=40) or MFX treatment alone (n=40). SVF were isolated 1 day before the MFX from the patient’s buttock was collected and stromal vascular fraction enzymatic digestion. ADSC characterisation in isolated SVF was also performed. SVF was administered under arthroscopic guidance after MFX procedure. Significantly better signal intensity was observed for the repair tissue in receiving MFX and ADSC than those on MFX alone. At a mean clinical follow-up of 27.4 months, compared to MFX alone, MFX and ADSCs with fibrin glue provided radiologic and KOOS pain and symptom subscore improvements with no differences in activity, sports, or quality-of-life subscores with similar structural repair tissue. Quantitative and qualitative assessments of the repair tissue at 24 months using the Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) scoring system with follow-up MRI showed that patients who were treated with MFX and ADSCs had superior MOCART scores compared with those treated with MFX alone.
Treatment of achilles tendinopathy: de Girolamo et al. [18] randomly allocated the patients with Achilles tendinopathy either to single PRP injection group (GPSIII kit, Biomet, USA) (n=28 tendons) or single adipose tissue SVF (FastKit, Corios, Italy) (n=28 tendons) injection group [18]. An aliquot of SVF of each patient was analysed in vitro for Mesenchymal Stem Cells (MSC) content, viability, proliferation rate, differentiation potential and immunomodulatory ability. Both treatments resulted into significant improvement in VAS Pain, VISA-A, AOFAS and SF-36 scores at 180 days as compared to baseline. MRI and ultrasound findings were in accordance with clinical outcomes. Notably, in patients receiving SVF, the improved scores from baseline were evident from 15 days after treatment and were statistically greater as compared to those receiving PRP injections. There were no side effects.
Efficacy and safety of MFAT for treatment of musculoskeletal disorders
We identified 5 studies that assessed efficacy and safety of MFAT/MF for treatment of musculoskeletal disorders. Three studies assessed safety and efficacy of MFAT/MF for treatment of OA. One RCT assessed efficacy and safety of MFAT for each, cartilage defects and Temporomandibular Joint (TMJ) disease.
Treatment of OA: Kaszyński evaluated efficacy of MFAT for treating OA compared to Leucocyte-Poor Platelet-Rich Plasma (LP-PRP) in an assessor-blind RCT. In this study, 54 OA patients (Kellgren–Lawrence OA grades I/II/III) were randomly allocated to receive multiple LP-PRP injections (n=28; three injections of intraarticular LP-PRP were given half a month apart in the affected knee) or a single intra-articular MFAT injection (MFAT was isolated using Lipogems; n=11) [19]. A statistically significant improvement was seen in the subjective evaluation parameters including VAS, KOOS, WOMAC, IKDC 2000 and EQ-5D-5L scales in both treatment arms at different time points compared to baseline. Treatment with LP-PRP and MFAT resulted in the significant improvement of functional assessments parameters, namely Timed Up and Go Test (TUG), the 5 Times Sit to Stand Test (5×STS) and the 10m Walk Test (10mWT). However, greater improvement in minimal detectable change was noted only in those treated with MFAT. Both treatments improved MVIC with time. However, the effect was apparent at 3-month follow-up with MFAT treatment whereas it was observed after 1 month with PRP treatment.
Another prospective, non-blinded randomized trial was carried out to assess the clinical efficacy of repeated doses of LP-PRP plus HA for treating early-stage OA was compared with a single dose autologous MFAT injection [20]. In this study, 50 patients (total 80 knees) were randomized to receive either combination of LPPRP and HA (n=25 patients) or MFAT (n=25 patients). Adipose was harvested from the abdomen and MFAT was isolated using the Lipogems device. While assessing the clinical efficacy using patientreported outcome measure scores at 6- and 12-months, both treatments were able to improve clinical and functional outcomes as evident by significant improvement in KOOS, VAS and Knee Measure, and Tegner scoring systems. However, better functionality was observed in patients treated with MFAT as evident by higher Tegner score and KOOS symptoms score (P<0.05) at 6 months. Twelve-month clinical outcomes favored to MFAT based on/ according to Tegner scores. No serious AEs were recorded during the follow-up.
Louis et al. [21] conducted comparative study to determine if adding platelet-rich-plasma to microfat (MF, defined as small lobules of fat (600μm) for treatment of knee OA would improve clinical and radiological outcomes for up to 6 months as compared to MF alone [21]. A total of 30 knee OA patients (KL grade II to IV) were randomised to receive single injection of MF mixed with saline (MF/Saline group; n=10) or MF mixed with a low-dose of pure PRP (1 billion of platelets; MF/PRP LD group; n=10) or MF mixed with a high dose of pure PRP (3 billion platelets; MF/PRP HD group; n=10). The MF was isolated from lipoaspirate using Puregraft 50 (Bimini, Solana Beach, CA). At 6-month follow-up, there was significant improvement in clinical outcomes in all three treatment groups as measured by WOMAC score, VAS score and knee joint range of motion compared to baseline. However, no statistical significant difference was seen between these treatment groups. Quantitative volumetric assessment of cartilage as measured by T2max demonstrated significant improvement only in the MF/ saline group at 6 months compared to baseline. Notably, except one patient who was treated with MF/saline, none of the patients in all three treatment groups attained clinically relevant change in T2max/clinically relevant quantitative volumetric improvement in cartilage. No difference was observed between treatment groups with regard to other MRI parameters (patients with increase of ≥0.05cm joint spacing, number of areas with improvement) at 6 months follow-up. Hence, 6-month follow-up of the study population demonstrated clinical efficacy of MF with or without PRP for treatment of knee OA. However, the study did not show superiority of PRP associated MF over MF alone. Further, there was no evidence of objective improvement of damaged cartilage.
Treatment of cartilage: A prospective randomized controlled, single blind clinical trial was designed to evaluate clinical efficacy of MFAT in combination with microfracture for treatment of symptomatic focal chondral lesion in comparison with microfracture alone [22]. In this study, 40 patients with a symptomatic focal chondral lesion of a femoral condyle (Outerbridge classification grades III/IV) were treated with either microfracture alone or MFAT along with microfractures. Margins of the cartilage lesions were debrided with a shaver. MFAT was isolated using Lipogems® (Lipogems International SpA, Milan, Italy) and injected under arthroscopic guidance. Both treatments were found to be efficacious and there was no difference in Consumption of analgesics and antiinflammatories between two treatments. Nonetheless, MFAT in combination with microfracture was found to be more effective than microfracture alone in terms of WOMAC, Oxford knee score, EQ-5D, VAS for pain and satisfaction with a medium effect size and these clinical benefits were evident from 6 months after treatment and were maintained for up to 1 year. Further, significantly greater improvement in pain could be seen 3 months after treatment in patients treated with MFAT. There were no adverse events related to MFAT; one patient who were treated with microfracture alone experienced knee effusion 3 days after surgery.
Treatment of TMJ diseases: Internal derangement and osteoarthritis are the most common degenerative TMJ diseases. As the research demonstrated clinical efficacy of MFAT for treatment of knee OA, Sembronio et al. [23] performed RCT to assess efficacy of intra-articular injection of autologous micro-fragmented adipose tissue along with arthrocentesis for treatment of TMJ internal derangement and osteoarthritis in comparison with HA with arthrocentesis [23]. In this study, 40 patients were randomly allocated to receive either micro-fragmented adipose tissue (n=20 patients; 5 bilateral and 15 unilateral) or HA (n=20 patients; 6 bilateral and 14 unilateral) after arthrocentesis process. Adipose tissue was harvested from abdomen and MFAT was isolated using the Lipogems system. Both treatments were found efficacious in reducing pain and improving TMJ functions throughout 6 months follow-up. However, MFAT treatment found more efficacious with regard to reduction in pain (at different time-points) and improvement in TMJ function (at 6-month follow-up). Similarly, at 6-month follow-up, the success of treatment, defined as maximum interincisal opening ≥35mm and VAS scale ≤2, was found to be higher among patients treated with MFAT was higher as compared to those treated with HA. There was no incidence of AE related to the joint procedures and to the lipoaspiration.
Discussion
Adipose tissue derived mesenchymal stem cells have been investigated in variety of clinical conditions, ranging from highimpact life-threatening diseases to chronic painful pathologies. However, cultured ADSCs possess significant challenges to clinic application due to extensive culturing periods and regulatory burden. Hence, research has been directed to explore the regenerative capabilities of uncultured ADSCs, MFAT and SVF. These approaches are more cost-effective and less labour intensive in comparison to cultured ADSCs. Further, evidence also suggests that uncultured ADSCs are superior to cultured ADSCs for tendon and bone regeneration [24,25]. Hence, a growing number of trials have begun to investigate efficacy of SVF and MFAT. till date, a total 12 RCTs are published that assessed efficacy of SVF/ MFAT in comparison with placebo or active arm for treatment of musculoskeletal diseases. It should be noted that majority of these studies compared efficacy of SVF/MFAT with other orthobiologics (HA, PRP) which themselves do not have standardised protocol for treatment of musculoskeletal diseases. Further, none of the RCT, till date, compares SVF/MFAT and BMMSCs (bone marrow aspirate or bone marrow concentrate).
Overall, the study demonstrated improved clinical outcomes
with MFAT/SVF based treatments. The included studies did not
have a uniform subjective outcome measures. However, they all
reported statistically significant improvements in at least one of
the parameters (WOMAC, VAS, KOOS, ROM, TUG, Tegner, Marx,
IKDC 2000, EQ-5D-5L, 10mWT, MVIC, VISA-A, AOFAS and SF-36)
at one time point compared to baseline. The difference in positive
outcomes is attributed by the presence of various confounding
variables. The confounding variables were as follow:
i. In the included studies, there was no standardised
protocol for SVF/MFAT-based treatment. Hence, there were
variations in harvest site, processing of harvested lipoaspirate
to isolate SVF/MFAT, delivery method, dosage, implementation
of guidance techniques during implantation procedure. Further,
histology, cytology and biochemical analysis of ADSCs and
growth factors that may affect outcomes were assessed only in
few studies;
ii. In majority of the trials, patients could not be truly blinded
as fat harvesting in the comparator arm (who were treated with
other treatment modalities) was not clinically relevant;
iii. There was heterogeneity in the study population with
respect to disease severity;
iv. In several studies, SVF/MFAT-based treatments were
performed in association with other intervention such as
debridement, chondral shaving and meniscectomy which itself
may provide beneficial effects.
Except four studies, all the studies (n=7 studies of SVF; n=1 study of MFAT) performed imaging evaluation of cartilage during follow-up period. However, imaging outcome measures were inconsistent across studies. Six studies reported improvement in imaging parameters whereas two studies reported no improvement in cartilage quality or thickness measured on MRI. Additionally, although there were significant improvements in clinical outcomes which were majorly subjective, imaging outcome measures were not always in accordance with them. Only one study performed histopathological evaluation and second-look arthroscopy of cartilage and there was no change in cartilage repair despite significant improvement in clinical outcomes and MOCART score with SVF treatment [26,27]. Except one RCT, all the studies described analgesic efficacy and functional improvement with SVF or MFAT treatments at short-term follow-up, ranging from 6 to 24 months period. However, orthopaedic degenerative diseases progress over years and thereby signs and symptoms reappear gradually. Further, conservative treatments are also able to produce short-term benefits. In these circumstances, it is imperative to assure that initial clinical benefits with SVF or MFAT or other orthobiologics continue to remain for a longer period of time.
Conclusion
None of the studies included in this review reported any serious treatment-related AEs. The most common reported AEs were Pain and swelling at the injection or harvest site which resolved within a few days. Hence, RCTs demonstrated that SVF and MFAT (within dose range) seem to be safe. The aforementioned observations demand standardization in terms of clinical and imaging outcome measures, and the SVF/MFAT based treatment protocol including harvest sites, dosage and mode of delivery of treatment and long-term follow-up. Designing future clinical trials or registries incorporating these parameters would be a clear step to achieve optimal clinical outcomes with MFAT/SVF for treatment of musculoskeletal disorders.
Limitations
The inherent limitations of the study were that the search was limited to only PubMed, and only articles in English language were screened..
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- Liu S, Wang B, Fan S, Wang Y, Zhan Y, et al. (2022) Global burden of musculoskeletal disorders and attributable factors in 204 countries and territories: A secondary analysis of the Global Burden of Disease 2019 study. BMJ Open 12(6): e062183.
- Li WJ, Jiao H, Walczak BE (2019) Emerging opportunities for induced pluripotent stem cells in orthopaedics. J Orthop Translat 17: 73-81.
- Furia JP, Lundeen MA, Hurd JL, Pearce DA, Alt C, et al. (2022) Why and how to use the body's own stem cells for regeneration in musculoskeletal disorders: A primer. J Orthop Surg Res 17(1): 36.
- Muthu S, Jeyaraman M, Jain R, Gulati A, Jeyaraman N, et al. (2021) Accentuating the sources of mesenchymal stem cells as cellular therapy for osteoarthritis knees-a panoramic review. Stem Cell Investig 8: 13.
- Zhang J, Liu Y, Chen Y, Yuan L, Liu H, et al. (2020) Adipose-derived stem cells: Current applications and future directions in the regeneration of multiple tissues. Stem Cells Int 2020: 8810813.
- Jayaram P, Ikpeama U, Rothenberg JB, Malanga GA (2019) Bone marrow-derived and adipose-derived mesenchymal stem cell therapy in primary knee osteoarthritis: A narrative review. PM R 11(12): 177-191.
- De Francesco F, Gravina P, Busato A, Farinelli L, Soranzo C, et al. (2021) Stem cells in autologous microfragmented adipose tissue: Current perspectives in osteoarthritis disease. Int J Mol Sci 22(19): 10197.
- Ding W, Xu Y, Zhang Y, Li A, Qiu X, et al. (2020) Efficacy and safety of intra-articular cell-based therapy for osteoarthritis: Systematic review and network meta-analysis. Cartilage 13(1): 104S-115S.
- Ha CW, Park YB, Kim SH, Lee HJ (2019) Intra-articular mesenchymal stem cells in osteoarthritis of the knee: A systematic review of clinical outcomes and evidence of cartilage repair. Arthrosc 35: 277-288.
- Jeyaraman M, Muthu S, Ganie PA (2021) Does the source of mesenchymal stem cell have an effect in the management of osteoarthritis of the knee? Meta-analysis of randomized controlled trials. Cartilage 13: 1532s-1547s.
- Hurley ET, Yasui Y, Gianakos AL, Seow D, Shimozono Y, et al. (2018) Limited evidence for adipose-derived stem cell therapy on the treatment of osteoarthritis. Knee Surg Sports Traumatol Arthrosc 26(11): 3499-3507.
- Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, et al. (2020) Clinical efficacy of intra-articular mesenchymal stromal cells for the treatment of knee osteoarthritis: A double-blinded prospective randomized controlled clinical trial. Am J Sports Med 48(3): 588-598.
- Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, et al. (2021) Comparison of clinical and imaging outcomes of different doses of adipose-derived stromal vascular fraction cell treatment for knee osteoarthritis. Cell Transplant 30: 9636897211067454.
- Hong Z, Chen J, Zhang S, Zhao C, Bi M, et al. (2019) Intra-articular injection of autologous adipose-derived stromal vascular fractions for knee osteoarthritis: A double-blind randomized self-controlled trial. Int Orthop 43(5): 1123-1134.
- Zhang S, Xu H, He B, Fan M, Xiao M, et al. (2022) Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: A minimum 5-year follow-up study. Stem Cell Res Ther 13(1): 105.
- Zhang Y, Bi Q, Luo J, Tong Y, Yu T, et al. (2022) The effect of autologous adipose-derived stromal vascular fractions on cartilage regeneration was quantitatively evaluated based on the 3D-FS-SPGR sequence: A clinical trial study. Biomed Res Int 2022: 2777568.
- Koh YG, Kwon OR, Kim YS, Choi YJ, Tak DH (2016) Adipose-derived mesenchymal stem cells with microfracture versus microfracture alone: 2-year follow-up of a prospective randomized trial. Arthroscopy 32(1): 97-109.
- Girolamo L, Grassi M, Viganò M, Orfei CP, Montrasio UA, et al. (2016) Treatment of achilles tendinopathy with autologous adipose-derived stromal vascular fraction: Results of a randomized prospective clinical trial. Orthop J Sports Med 4(7): 1-1.
- Kaszyński J, Bąkowski P, Kiedrowski B, Stołowski Ł, Wasilewska-Burczyk A, et al. (2022) Intra-articular injections of autologous adipose tissue or platelet-rich plasma comparably improve clinical and functional outcomes in patients with knee osteoarthritis. Biomedicines 10(3): 684.
- Dallo I, Szwedowski D, Mobasheri A, Irlandini E, Gobbi A (2021) A prospective study comparing leukocyte-poor platelet-rich plasma combined with hyaluronic acid and autologous microfragmented adipose tissue in patients with early knee osteoarthritis. Stem Cells Dev 30(13): 651-659.
- Louis ML, Dumonceau RG, Jouve E, Cohen M, Djouri R, et al. (2021) Intra-articular injection of autologous microfat and platelet-rich plasma in the treatment of knee osteoarthritis: A double-blind randomized comparative study. Arthrosc 37(10): 3125-3137.
- Bisicchia S, Bernardi G, Pagnotta SM, Tudisco C (2020) Micro-fragmented stromal-vascular fraction plus microfractures provides better clinical results than microfractures alone in symptomatic focal chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 28(6): 1876-1884.
- Sembronio S, Tel A, Tremolada C, Lazzarotto A, Isola M, et al. (2021) Temporomandibular joint arthrocentesis and microfragmented adipose tissue injection for the treatment of internal derangement and osteoarthritis: A randomized clinical trial. J Oral Maxillofac Surg 79(7): 1447-1456.
- Nyberg E, Farris A, O'Sullivan A, Rodriguez R, Grayson W (2019) Comparison of stromal vascular fraction and passaged adipose-derived stromal/stem cells as point-of-care agents for bone regeneration. Tissue Eng Part A 25(21-22): 1459-1469.
- Polly SS, Nichols AEC, Donnini E, Inman DJ, Scott TJ, et al. (2019) Adipose-derived stromal vascular fraction and cultured stromal cells as trophic mediators for tendon healing. J Orthop Res 37(6): 1429-1439.
- Oberbauer E, Steffenhagen C, Wurzer C, Gabriel C, Redl H, et al. (2015) Enzymatic and non-enzymatic isolation systems for adipose tissue-derived cells: Current state of the art. Cell Regen 4: 7.
- Mazini L, Ezzoubi M, Malka G (2021) Overview of current Adipose-Derived Stem Cell (ADSCs) processing involved in therapeutic advancements: Flow chart and regulation updates before and after COVID-19. Stem Cell Res Ther 12(1): 1.
© 2024 Katz Nathan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






