- Submissions

Full Text
Evolutions in Mechanical Engineering
Treatment of Industrial Wastewater through the Process of Electrocoagulation: A Review
Mahnoor, Aoha Roohi Amin*, Muhammad Zeeshan and Tuba Safdar
Department of Chemical and Energy Engineering, Institute of Applied Sciences and Technology (PAF-IAST), Pakistan
*Corresponding author:Aoha Roohi Amin, Department of Chemical and Energy Engineering, Institute of Applied Sciences and Technology (PAF-IAST), Pakistan
Submission: October 25, 2023;Published: November 28, 2023

ISSN 2640-9690 Volume5 Issue1
Abstract
Several different approaches are employed for wastewater treatment to reduce the quantity of harmful substances that are let out into the surroundings each year from a variety of industries. By electrochemically dissolving sacrificial anodes, often formed of iron or aluminum, the Electrocoagulation (EC) technique destabilizes contaminants that are dissolved, suspended, or emulsified. It has the capacity to eliminate a range of pollutants from various types of wastewaters, including organic and inorganic contaminants. The pH, electrode, operating period and current density are only a few of the variables that affect how well the EC process works. Reviewing the most pertinent newly published material is the aim of this study. The electrode passivation and energy use issues with the EC technique are the key difficulties. EC has benefits over other conventional technologies, including lower operational expenses and lower energy use. A survey of the literature covering electrocoagulation electrochemical treatment of wastewater published during the previous 32 years was given. The review discusses issues about electrocoagulation, the background electrocoagulation hybrid process for industrial and leachate wastewater treatment and its cost effectiveness.
Keywords: Electrocoagulation; Electrodes; Wastewater treatment
Introduction
Today, the detrimental effects of contaminated water and wastewater, as well as the depletion of water resources, are undeniable global problems. The most significant environmental issues of the twenty-first century are pollution and water recycling. Industrial wastewater is defined in a very broad sense and differently from home wastewater. The effluent’s composition and characterization are entirely varied and extremely complex because of the numerous types of industries and application processes [1]. Industrial effluent can be classified into different categories depending on the harm it causes to the environment [2]. Electrocoagulation (EC) has been used effectively as a first treatment in the elimination and modification of polycyclic aromatic hydrocarbons from industrial effluents [3,4]. The Fe or Al anode is oxidized during wastewater treatment electrolysis, yielding corresponding metal ions that instantly hydrolyze to polymeric iron or aluminium hydroxide. These polymeric hydroxides are good coagulants, and the tiny oxygen and hydrogen bubbles created by the anode and cathode may help in particle flocculation in the wastewater [1]. It should be noted that water treatment methods seem to be the most effective way to lessen the impact of pollution on aquatic and aqueous systems. Every wastewater treatment facility tries to address the previously mentioned environmental issues. Physical-chemical treatments are the most common type of treatment.
Since they have been used for producing drinkable water for people for ages [5]. The harmful substances found in wastewater are now entirely distinct and complex because of industrial operations and technological advancement. As a result, research into water treatment techniques has been crucial to treating the growing pollution. Table 1 shows the breakdown of dangerous compounds found in industrial effluent and their likely sources. [6,7]. Many traditional methods/units are used to treat industrial water, coagulation has fewer advantages and disadvantages, so scientists introduce the hybrid method called electrocoagulation, which is more effective and reasonable than coagulation [6]. Electrocoagulation (EC) has been used effectively as a first treatment in removing and transforming polycyclic aromatic hydrocarbons from industrial effluents. EC is a chemical and physical technique that injects ions into wastewater using consumable electrodes such as Fe or Al [8]. The Fe or Al anode is oxidized wastewater treatment electrolysis, yielding corresponding metal ions that instantly hydrolyze to polymeric iron or aluminium hydroxide. These polymeric hydroxides are good coagulants, and the tiny oxygen and hydrogen bubbles created by the anode and cathode may help in particle flocculation in the water [9] (Table 2).
Table 1: Contaminants in wastewater and their potential sources.
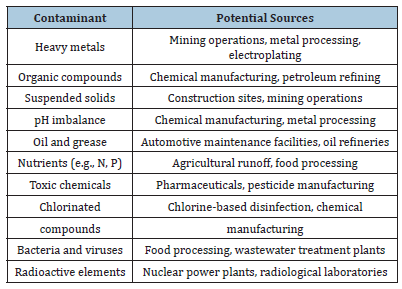
Table 1: Conventional operational units and their description (Yusmartini et al. [19]).
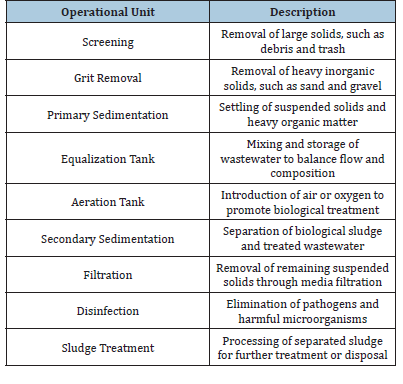
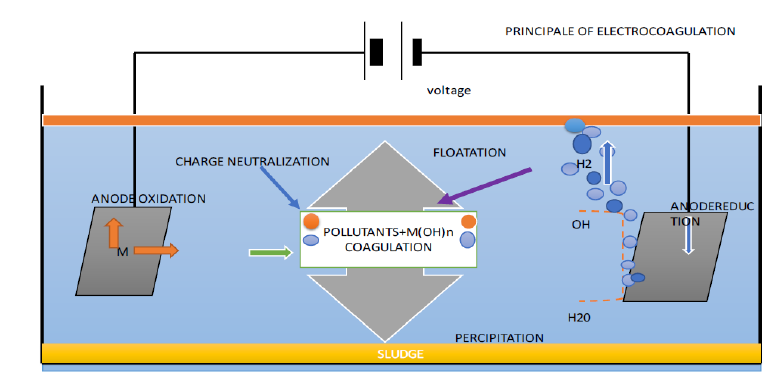
This review article aims to focus on the EC affecting factors in the cripples & fundamentals of the EC process, theory related to its mechanism and potential applications in different areas. Al+3(aq) and OH- (aq) ions produced by reactions at electrodes and form numerous monomeric species, which changes finally into Al(OH)3 by complex precipitation kinetics [10]. The electrostatic antiparticle repulsion is reduced to the point that van der Waals attraction takes hold, resulting in coagulation. There is no net fee because of the procedure. (C) Flocs formation: Colloidal particles that remain in the aqueous medium are trapped and bridged by the sludge blanket created by merged flocs. In a parallel process, water is electrolyzed to produce small bubbles of hydrogen at the cathode and oxygen at the anode. [11]. By drawing flocculated particles to them, these bubbles cause the pollutants to rise to the surface due to their inherent buoyancy. More effective coagulants than those used in chemical dosing are hydroxides, ox hydroxides, and polymeric hydroxides. They can precipitate or adsorb dissolved contaminating species, destabilize colloidal suspensions and emulsions, and create flocs that may be removed by flotation or settling/filtration [12] (Figure 1).
Figure 1:Experimental Setup of Electrocoagulation process.
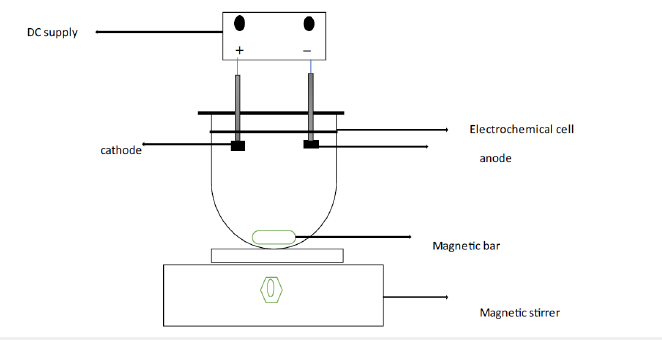
Principles of electrocoagulation
An electrochemical cell is used in the EC procedure to treat the water. An electrochemical cell’s main components are the anode and the cathode, which are dissolved in an electrolyte or conducting solution and connected by an electrical circuit to provide a current source and a control device [5]. The anode’s metallic cations hydrolyze to generate hydroxides, poly hydroxides and poly hydroxyl metallic compounds with a strong attraction for scattered particles and counter ions, causing coagulation. By decreasing the repulsive potential of the electrical double layer, they may decrease the net surface charge of colloidal particles in suspension [13]. Therefore, the repulsive interactions between colloidal particles weaken, bringing the particles together to the point where van der Waals forces prevails and particle agglomeration occurs. It should be noted that, unlike chemical coagulation, the processes of flocculation and coagulation in EC happen simultaneously and cannot be differentiated from one another. The two processes, flocculation and coagulation, are physically separated or differentiated when metal salts are used in water treatment facilities based on the amount of time required for each (“An Overview of the EC Process for the Treatment of Wastewater,” 2018) [14] (Figure 2).
Figure 2:Main principle of EC Procedure.
Fundamentals of electrocoagulation and oxidation (Electrocoagulation Oxidation (ECO))
A comprehensive understanding of the fundamentals is crucial to comprehend the underlying mechanisms of ECO. Electrocoagulation (EC) involves destabilizing and coagulating pollutants present in wastewater by applying an electric current [15]. The process typically takes place in an electrolytic cell, where the wastewater acts as the electrolyte. When a direct current is applied, metal species (such as aluminium or iron) are released from the electrodes, generating metal hydroxide species. These species act as coagulants and aid in removing pollutants through coagulation, flocculation, and sedimentation processes [16]. Several important variables, such as the electrode material, current density, pH, and treatment duration, affect how successful EC is. The kind and concentration of metal ions emitted during electrocoagulation are determined by the electrode material chosen, therefore choosing wisely is essential [17]. Iron and aluminum are frequently utilized as electrode materials due to their affordability, availability, and effectiveness in generating coagulants. However, other materials such as titanium, stainless steel, and graphite have also been investigated for their suitability in specific applications [18].
In addition to electrocoagulation, integrating oxidation processes with EC has attracted a lot of attention lately, leading to the development of Electrocoagulation Oxidation (EC0) as an advanced treatment approach. Incorporating oxidation techniques aims to increase the degradation and mineralization of recalcitrant pollutants that are not effectively removed through traditional electrocoagulation alone [19]. Electrochemical oxidation is a widely explored technique that utilizes the electrochemical generation of most reactive species to degrade organic compounds in wastewater. Commonly employed anodes for electrochemical oxidation include Boron-Doped Diamond (BDD), Mixed Metal Oxide (MMO), and Platinum Group Metal (PGM) electrodes [20]. These anodes facilitate the amount of Reactive Oxygen Species (ROS), potent oxidants capable of breaking down complex organic molecules into more direct, less harmful by-products. Several studies have demonstrated electrochemical oxidation’s effectiveness in degrading organic dyes, pharmaceuticals, and other persistent pollutants [21].
Another promising oxidation technique combined with electrocoagulation is photo electrochemical oxidation. This approach utilizes the synergistic effects of light and electrochemical processes to enhance the degradation of pollutants. Typically, a photoactive semiconductor material, such as titanium dioxide (TiO2), is incorporated into the EC system [22]. When illuminated with UV or visible light, the photo excited electrons and holes generated on the surface of the semiconductor promote redox reactions, facilitating the degradation of organic pollutants. Photo electrochemical oxidation has shown promising results in treating various organic contaminants, including pesticides and emerging micro pollutants [21]. Sono-electrocoagulation is another innovative approach that combines the application of ultrasound with electrocoagulation. Ultrasound waves generate cavitation, leading to micro bubbles forming and collapsing in the wastewater [23]. The collapse of these micro bubbles produces shockwaves and localized high temperatures and pressures, creating an environment conducive to oxidation and enhancing pollutant degradation [24].
Numerous investigators have made noteworthy contributions to the comprehension and progression of EC0 technology. To achieve high removal efficiency, for example, a thorough analysis was carried out on the use of EC0 to treat pharmaceutical effluent. Their research emphasized the role that optimization factors have on EC0 system performance [20]. Furthermore Sari et al. [25] centered on treating wastewater containing dyes by combining photo electrochemical oxidation and electrocoagulation. Their study showed how the integration of light- responsive semiconductor materials might facilitate effective electron-hole separation and subsequent pollutant oxidation, hence optimizing the degradation of dyes by photo electrochemical oxidation. Additionally, Chakchouk et al. [22] examined how well sono-electrocoagulation worked to remove organic pollutants from wastewater. Comparing their study to traditional electrocoagulation, they found that the combined effects of ultrasound and electrocoagulation led to higher rates of pollutant breakdown. The researchers emphasized the importance of optimizing process parameters, such as applied current, pH, and ultrasonic frequency, to maximize the sono-electrocoagulation efficiency (Figure 3).
Figure 3:Flowchart Showing Electrocoagulation treatment.
Applications of Electrocoagulation
Wastewater treatment
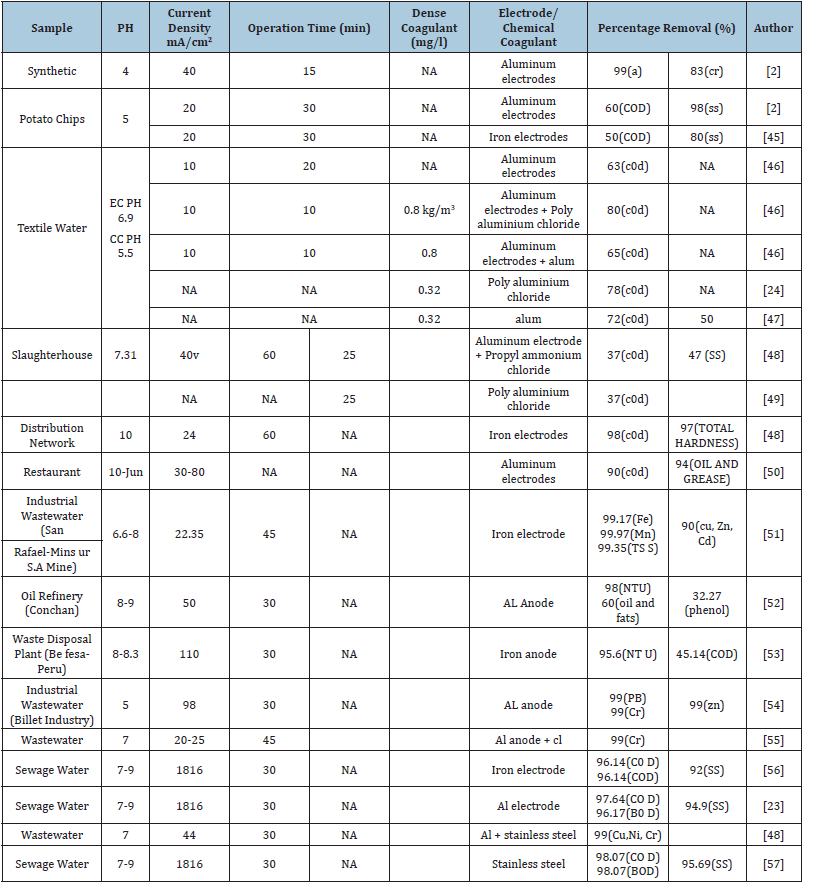
Wastewater treatment is a critical area where Electrocoagulation (EC) has gained significant attention due to its effective removal of contaminants. This section reviews the work conducted by different researchers in exploring the potential applications of electrocoagulation. These researchers use different electrodes to examine which electrode has more removal efficiency and are costlier. Some researchers, like El-Ashtoukhy et al. [26], examined the use of EC to remove heavy metals from industrial wastewater and emphasized the significance of optimization factors like pH and current density Ravadelli et al. [27] explored the application of EC for dye-containing wastewater treatment, focusing on the influence of electrode material, current density, and initial dye concentration An et al. [28] studied the treatment of oil-water emulsions using EC and investigated vital factors Zodi et al. [6] conducted a comprehensive review of EC for industrial wastewater treatment, covering mechanisms, electrode materials, optimization parameters and performance evaluation Gil Pavas et al. [29] examined the removal of organic pollutants from wastewater using a combination of EC and activated carbon adsorption, highlighting the synergistic effects of the two processes Zaied et al. [12] investigated the removal of pharmaceutical compounds from wastewater using EC and discussed the factors affecting their removal efficiency. Ammar et al. [30] explored the application of EC for treating petroleum refinery wastewater, focusing on removing oil, suspended solids, and heavy metals Huda et al. [31] studied landfill leachate treatment using EC and evaluated the influence of electrode materials, current density, and initial leachate characteristics on the process efficiency Parga et al. [32] investigated the removal of arsenic from groundwater using EC and discussed the impact of operational parameters on arsenic removal efficiency. Moussa et al. [33] examined turbidity removal from water using EC and analyzed the effects of various factors, including current density, electrode spacing, and water pH Bilińska et al. [34] focused on treating textile wastewater using EC and evaluated the influence of electrode material, current density, and initial pollutant concentration on the removal efficiency. Alaton et al. [35] studied the removal of organic dyes from wastewater using EC and discussed the effects of process parameters on decolorization efficiency.
Galvão et al. [36] explored the application of EC for treating landfill leachate and discussed the removal efficiency of pollutants such as COD, ammonia and heavy metals. Phalakornkule et al. [37] investigated the removal of Total Organic Carbon (TOC) from industrial wastewater using EC. They discussed the effects of electrode material and current density on TOC removal efficiency. Zheng [9], Ammar et al. [38] explored the application of EC to treat oilfield-produced water and discussed the removal efficiency of oil, suspended solids and heavy metals. Song et al. [39] investigated the removal of arsenic from groundwater using EC and discussed the influence of operational parameters on nitrate removal efficiency. Thakur et al. [7] studied dye wastewater treatment using EC and evaluated the effects of electrode material, current density, and electrolyte concentration on decolorization efficiency. In addition to the studies mentioned above, numerous other research papers have contributed to the understanding and advancement of electrocoagulation for wastewater treatment. These studies have explored various aspects, such as pollutant removal mechanisms, optimization parameters, electrode materials, and treating specific wastewater types. Collectively, these works provide valuable insights into the applications and potential of electrocoagulation as an efficient and sustainable wastewater treatment technology. The Table 3 given below highlights the key points of other researchers.
Table 3: Application and effectiveness of electrocoagulation process for different industrial wastewater.
Leachate treatment
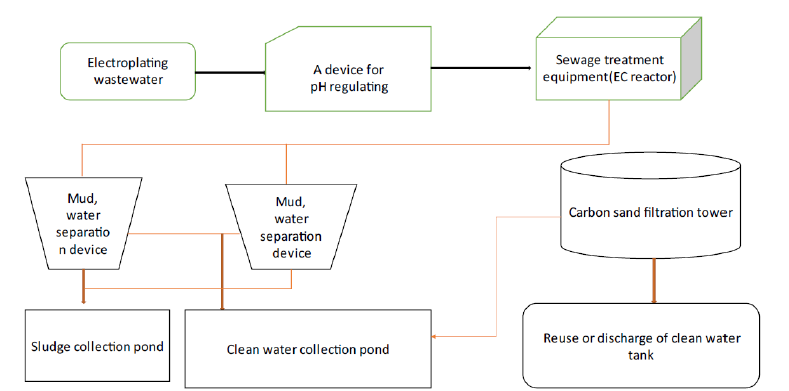
Landfilling is the most prevalent and convenient method of disposing of solid waste. The dump often receives garbage from municipalities close to the site. If the site of trash generation is distant from the transfer station, there is a method to cut garbage transportation costs. In most cases, waste items from residential, business, and institutional areas were mixed. Landfills produce three types of outputs: gas, liquid (leachate), and inert solids [40]. Leachate is difficult to treat due to its complicated structure and high pollutant load. They become the primary pollutant of wastewater because it is the most difficult to treat due to the complex and wildly varied content created inside landfills [41]. Many different sorts of contaminants may be found in leachate wastewater. Biochemical Oxygen Demand (BOD), Chemical Oxygen Demand (C0D), ammonia and high concentrations of several metals are indicators of complex pollution. The presence of these contaminants in high concentrations harms the ecosystem. As a result, many therapies and pre-treatments work together to prove that they treat Leachate [42]. Membrane processes, sophisticated oxidation techniques, coagulation- flocculation procedures, and lagoon and wetland applications are some well-known technologies used for leachate treatment. However, they are costly and difficult procedures, and a developing no tendered simple ones. Electrocoagulation is a simple technique for successfully treating wastewater [14].
Because of its great efficiency and cheap maintenance, this electrochemical therapy seems to be a potential therapeutic choice. Less effort is needed and results are obtained quickly (Table 4 & 5). For a particular pollutant, electrocoagulation may supply a choice for employee other chemical coagulation like astral salts or polymers. The electrode may produce coagulated species and metal hydroxide, which help to stabilize and agglomerate suspended particles [43]. The hydrogen gas emitted by the cathode, which aids in the flocculation of particles in water. The electrocoagulation technique is dependable and cost-effective, producing little sludge and showing no sensitivity to hazardous metals. A coagulant is produced by the electrolytic oxidation sacrificial cathode by using a direct current [44] (Figure 4).
Table 4:Leachate properties at different pH.
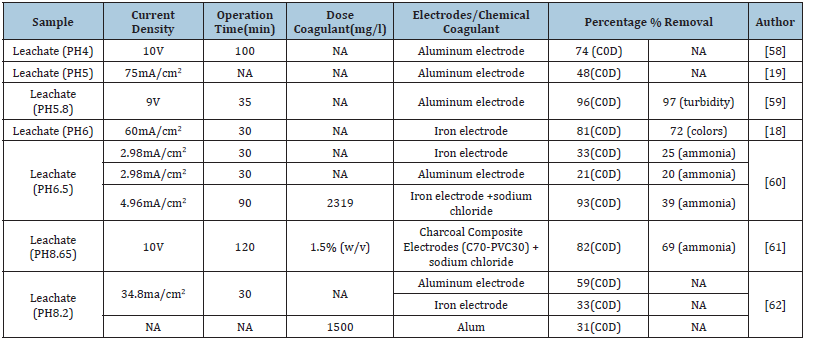
Table 5:Types of pollutant removal.
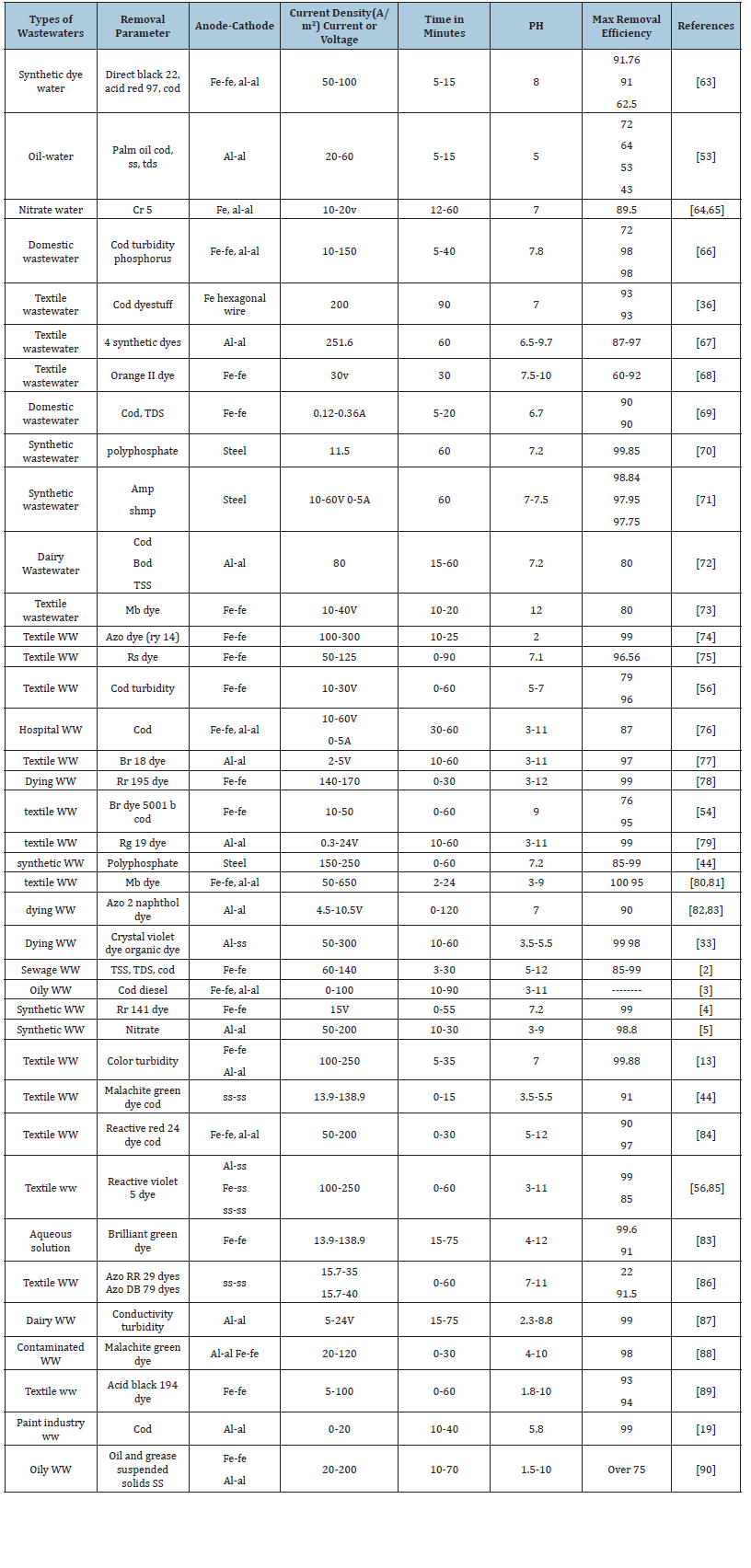
Figure 4:Setup for Leachate treatment.
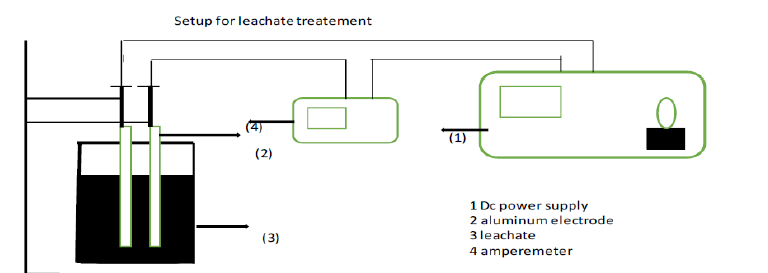
Factors affecting electro-coagulation
Several variables can alter how effective an EC is at removing impurities from wastewater [45-57]. These variables include the kind of power source, the spacing between the electrodes, the shaking speed, the length of the treatment, the density of the current given, the layout of the electrodes, and the material of the electrode selected [58-62].
Influnce of current density
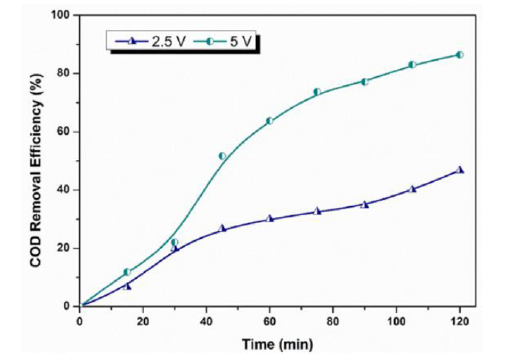
The electrode material is selected considering factors like the type of contaminant, expected results and chemical orientation of the contaminant present [63-75]. The electrode material not only affects the efficiency of the process but also affects the cost of the system[76-90]. Iron and Aluminum electrodes are extensively used for EC while they deionize into respective ions (Fe2 +, Fe3+,Al3 +). Several studies have been carried out to compare the Al and Fe electrodes [12,91]. Shen and co-workers while working on micro plastic removal reported a high removal efficiency of (>98.6%) for four different types of micro plastic including (polyethylene, polymethylmethacrylate, cellulose acetate [92]. As the electrocoagulation works on the Coulomb’s law of electrostatics, the distance between plates affects the electrostatic field and in turn the removal efficiency [68,93]. Proposed that an increase in the distance between the plates results in a greater removal of pollutants because the MOH ions collisions are hindered due to a little space between electrodes. While a greater agglomeration is possible when ions movement is slow and the possibility of flocs increases gradually (Figure 5). Applied current density is related to the generation of MOH ions and power consumption of the unit. An optimized current density investigation is thus required to operate the system. Galvão et al. [36]; Espinoza- Quinones et al. [67] observed a linear increase in the removal efficiency with increase in applied current density keeping all other parameters same [66] (Figure 6). The coagulant dose and bubble formation rate, the size and growth rate of the flocs, the bubble size, the reaction kinetics, and the energy consumption of the EC are all influenced by the applied current density [26,94]. There is a relationship between the voltage and the applied current density. In an electrochemical cell, higher voltages cause currents to rise and vice versa; but, in certain circumstances, such as when the electrodes are passivized, high over potentials cause currents to fall [70,95].
Figure 5:Effect of Al and Fe electrode on removal of micro plastics during electrocoagulation.
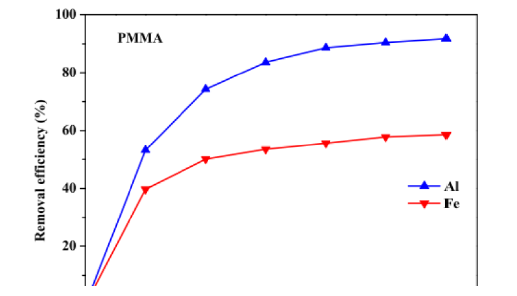
Figure 6:
Cost Analysis
With so many wastewater treatment alternatives, cost-effective electrocoagulation is required [96-106]. Kobya et al. discovered that 99.4% cadmium removal, 99.1% nickel removal, and 99.7% cyanide removal could be obtained from electroplating rinse water. The treatment cost $1.05/m3 for cadmium and $2.45/m3 for nickel and cyanide if the conditions were best. Remazol Red 3B decolorization using iron electrodes and discovered that under ideal conditions, 99% decolorization was possible. The authors discovered that 3.3 kWh/kg dye could be obtained for 0.6euro/m3 of energy consumption Chen X et al. [106] decided that fluorescent penetrant liquid may be treated with aluminum electrodes for nondestructive testing in the aviation sector. Using electrocoagulation, the current treatment achieved 95% (COD), 99% colour, and 99% turbidity. This more intensive course of treatment allowed for a 17-week payback period. (Sallam Mohammed et al. n.d.) conducted laboratory experiments and analyses on Oil Bilge Wastewater [OBW] using iron and aluminum electrodes in Bipolar (BP) and Monopolar (MP) configurations. Using best circumstances, electrocoagulation treatment of oil bilge effluent 93% biochemical oxygen demand, 95.6% oil and grease, 99.8% total suspended particles and 98.4% turbidity [107]. According to this analysis, the costs of oil bilge treatment were $0.46/m3 for energy and electrode consumption, chemicals, and sludge disposal.
Amorphous aluminum hydroxides, current densities, and electrode density were all considered in the electrochemical removal of iron [Fe(II)] from tap water using aluminum electrodes The authors discovered that treating a concentration of 15mg/L Fe(II) concentration would cost USD 6.05/m3 of tap water. Electrocoagulation with mild steel electrodes was used to treat agro-industry effluent (meat processing, cereal, and food drinks) [21]. In terms of Chemical Oxygen Demand (COD), 82% elimination was obtained at treatment costs ranging from $0.95 to USD 4.93/ m3, which formed electrical power, chemical, and electrode use. The application might be expanded to include the maritime sector. employed bipolar electrode configurations to remove 80% turbidity, 56% Chemical Oxygen Demand (COD), 90% oil and grease, and 89% Biochemical Oxygen Demand (BOD) from this sector using electrocoagulation-flocculation [22,108].
Advantages and disadvantages of electro-coagulation
Although the advantages of electrocoagulation and its achievement to resolve many environmental issues surpass its disadvantages, the two decades of research explains both. The disadvantages include high electricity consumption, especially in countries with high prices of electricity. The formation of oxide film leading to a decrease in removal efficiency [23] EC unable to treat wastewater with high electrolyte content due to the risk of a short circuit. Because of rust, the electrodes need to be changed on a regular basis. The development of layers on the electrodes that lower the electrocoagulation process’s efficiency [24,109]. There is no need for chemical coagulants or microbes in EC because the sacrificial anode itself releases ions that act as coagulants. When employed as a pretreatment procedure, EC can dramatically lower energy usage while raising water quality [110]. The treated water or effluent of the EC has its own advantages as it is used in agriculture, especially for irrigation purposes [111]. Low volumes of sludge are created in EC as opposed to chemical coagulation; this sludge can be used as building materials, fertilizers, pigments and absorbents [112,113]. The production of denser and hydrophilic sludge helps decantation and makes flotation easier. EC finds application in different pollutants removal such as heavy metals, dyes and microorganisms [34,114,115].
Discussion and Future Perspective
Different electrode materials resulted in varying percentages of COD, turbidity, and ammonia removal. According to the review, aluminium electrode outperforms iron electrode in terms of 90% removal of COD and suspended solids for different wastewater treatment. Furthermore, for leachate treatment, aluminium electrode outperforms iron electrode in terms of 85% removal effectiveness for colour, turbidity, and COD. One of the most crucial factors influencing EC performance is current density. Because of the creation of bubbles and flocs, high current density resulted in greater COD removal. Different electrode materials resulted in varying percentages of COD, turbidity, and ammonia removal. According to the review, aluminium electrode outperforms iron electrode in terms of 90% removal of COD and suspended solids for different wastewater treatment. Furthermore, for leachate treatment, aluminium electrode outperforms iron electrode in terms of 85% removal effectiveness for colour, turbidity, and COD. One of the most crucial factors influencing EC performance is current density. Because of the creation of bubbles and floc, high current density resulted in greater COD removal. Aside from that, pH is regarded as a crucial aspect in EC performance [40,115]. The cathode’s actions also have an impact on the pH value. Higher EC efficiency may be gained if the treatment procedure takes current density, electrode type, and pH into account. The cathode’s actions also have an impact on the pH value [7,54]. Higher EC efficiency may be gained if the treatment procedure takes current density, electrode type, and pH into account. Additionally, it might be used in combination with other treatment methods to improve performance [10]. This connected system may be used to disinfect drinking water. Other recent research areas not addressed here are the use of porous electrodes, Nano electrodes, and threedimensional electrodes. All things considered the EC process is thought to be a treatment technique that could get around a few obstacles in the process of removing heavy metals from industrial wastewater.
References
- Abbas SH, Ali WH (2018) Electrocoagulation technique used to treat wastewater: A review. American Journal of Engineering Research 7(10): 74-88.
- Abdelwahab O, Amin NK, El-Ashtoukhy EZ (2009) Electrochemical removal of phenol from oil refinery wastewater. Journal of Hazardous Materials 163(2-3): 711-716.
- Abdulhadi B, Kot P, Hashim K, Shaw A, Muradov M, et al. (2021) Continuous-flow Electrocoagulation (EC) process for iron removal from water: Experimental statistical and economic study. Science of the Total Environment 760: 143417.
- Adhikari B, Khanal SN (2015) Qualitative study of landfill leachate from different ages of landfill sites of various countries including Nepal. Journal of Environmental Science, Toxicology and Food Technology 9(1): 23-36.
- Sandi Afriani F, Tiandho Y (2020) Application of electrocoagulation for textile wastewater treatment: A review. Earth and Environmental Science 599.
- Zodi S, Potier O, Lapicque F, Leclerc JP (2010) Treatment of the industrial wastewaters by electrocoagulation: Optimization of coupled electrochemical and sedimentation processes. Desalination 261(1-2): 186-190.
- Thakur S, Chauhan M (2018) Treatment of dye wastewater from textile industry by electrocoagulation and Fenton oxidation: A review. Water Quality Management 79: 117-129.
- Zodi S, Potier O, Lapicque F, Leclerc JP (2009) Treatment of the textile wastewaters by electrocoagulation: Effect of operating parameters on the sludge settling characteristics. Separation and Purification Technology 69(1): 29-36.
- Zheng T (2017) Treatment of oilfield produced water with electrocoagulation: Improving the process performance by using pulse current. Journal of Water Reuse and Desalination 7(3) 378-386.
- Díaz CB, Uribe BF, Bilyeu B (2014) Removal of organic pollutants in industrial wastewater with an integrated system of copper electrocoagulation and electrogenerated H2O2. Chemosphere 105: 160-164.
- Azarian G, Rahmani AR, Atashzaban Z, Nematollahi D (2018) New batch electro-coagulation process for treatment and recovery of high organic load and low volume egg processing industry wastewater. Process Safety and Environmental Protection 119: 96-103.
- Zaied B, Rashid M, Nasrullah M, Zularisam A, et al. (2020) A comprehensive review on contaminants removal from pharmaceutical wastewater by electrocoagulation process. Science of the Total Environment 726: 138095.
- Akansha J, Nidheesh P, Gopinath A, Anupama K, Kumar MS (2020) Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 253: 126652.
- Akbal F, Camcı S (2011) Copper, chromium and nickel removal from metal plating wastewater by electrocoagulation. Desalination 269(1-3): 214-222.
- Liu F, Zhang C, Li H, Offiong NAO, Bi Y, et al. (2023) A systematic review of electrocoagulation technology applied for microplastics removal in aquatic environment. Chemical Engineering Journal 456: 141078.
- Louhıchı G, Bousselmı L, Ghrabı A, Khounı I (2019) Process optimization via response surface methodology in the physico-chemical treatment of vegetable oil refinery wastewater. Environmental Science and Pollution Research 26(19): 18993-19011.
- Kötz R, Stucki S, Carcer B (1991) Electrochemical wastewater treatment using high overvoltage anodes. Part I: Physical and electrochemical properties of SnO2 Journal of Applied Electrochemistry 21: 14-20.
- Maghanga J, Segor K, Irina J, Tole M (2017) Effect of process parameters on the electro coagulation of azo-dye wastewater in a Kenyan textile factory. IOSR J Appl Chem 10(11): 1- 7.
- Yusmartini ESY, Mardwita M, Aseptianova A (2021) Assistance and training of compost products from organic waste at SMA Negeri I Tanjung Raja, Ogan Ilir, South Sumatra. Asian Journal of Applied Research for Community Development and Empowerment 5(3): 43-47.
- Özyonar F, Korkmaz MU (2022) Sequential use of the electrocoagulation-electrooxidation processes for domestic wastewater treatment. Chemosphere 290: 133172.
- Chen G (2004) Electrochemical technologies in wastewater treatment. Separation and Purification Technology 38(1): 11-41.
- Chakchouk I, Elloumi N, Belaid C, Mseddi S, Chaari L, et al. (2017) A combined electrocoagulation-electrooxidation treatment for dairy wastewater. Brazilian Journal of Chemical Engineering 34(1): 109-117.
- Can O, Kobya M, Demirbas E, Bayramoglu M (2006) Treatment of the textile wastewater by combined electrocoagulation. Chemosphere 62(2): 181-187.
- Bukhari AA (2008) Investigation of the electro-coagulation treatment process for the removal of total suspended solids and turbidity from municipal wastewater. Bioresource Technology 99(5): 914-921.
- Sari MA, Chellam S (2015) Mechanisms of boron removal from hydraulic fracturing wastewater by aluminum electrocoagulation. Journal of Colloid and Interface Science 458: 103-111.
- El-Ashtoukhy EZ, Amin N, Fouad Y, Hamad H (2020) Intensification of a new electrocoagulation system characterized by minimum energy consumption and maximum removal efficiency of heavy metals from simulated wastewater. Chemical Engineering and Processing-Process Intensification 154: 108026.
- Ravadelli M, Da Costa RE, Lobo-Recio MA, Akaboci TRV, Bassin JP, et al. (2021) Anoxic/oxic membrane bioreactor assisted by electrocoagulation for the treatment of azo dye containing wastewater. Journal of Environmental Chemical Engineering 9(4): 105286.
- An C, Huang G, Yao Y, Zhao S (2017) Emerging usage of electrocoagulation technology for oil removal from wastewater: A review. Science of The Total Environment 579: 537-556.
- Gil Pavas E, Correa-Sanchez S (2020) Assessment of the optimized treatment of indigo-polluted industrial textile wastewater by a sequential electrocoagulation-activated carbon adsorption process. Journal of Water Process Engineering 36: 101306.
- Ammar SH, Ismail NN, Ali AD, Abbas WM (2019) Electrocoagulation technique for refinery wastewater treatment in an internal loop split-plate airlift reactor. Journal of Environmental Chemical Engineering 7(6): 103489.
- Huda N, Raman A, Bello M, Ramesh S (2017) Electrocoagulation treatment of raw landfill leachate using iron-based electrodes: effects of process parameters and optimization. Journal of Environmental Management 204(1): 75-81.
- Parga JR, Cocke DL, Valenzuela JL, Gomes JA, Kesmez M, et al. (2005) Arsenic removal via electrocoagulation from heavy metal contaminated groundwater in la comarca lagunera Mexico. Journal of Hazardous Materials 124(1-3): 247-254.
- Moussa DT, El-Naas MH, Nasser M, Al-Marri MJ (2017) A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. Journal of Environmental Management 186(1): 24-41.
- Bilińska L, Blus K, Gmurek M, Ledakowicz S (2019) Coupling of electrocoagulation and ozone treatment for textile wastewater reuse. Chemical Engineering Journal 358: 992-1001.
- Alaton IA, Gursoy BH, Akyol A, Kobya M, Bayramoglu M (2010) Modeling and optimization of acid dye manufacturing wastewater treatment with Fenton's reagent: Comparison with electrocoagulation treatment results and effects on activated sludge inhibition. Water Science and Technology 62(1): 209-216.
- Galvão N, De Souza JB, De Sousa Vidal CM (2020) Landfill leachate treatment by electrocoagulation: Effects of current density and electrolysis time. Journal of Environmental Chemical Engineering 8(5): 104368.
- Phalakornkule C, Mangmeemak J, Intrachod K, Nuntakumjorn B (2010) Pretreatment of palm oil mill effluent by electrocoagulation and coagulation. Science Asia 36(2): 142-149.
- Ammar SH, Akbar AS (2018) Oilfield produced water treatment in internal-loop airlift reactor using electrocoagulation/flotation technique. Chinese Journal of Chemical Engineering 26(4): 879-885.
- Song P, Yang Z, Zeng G, Yang X, Xu H, et al. (2017) Electrocoagulation treatment of arsenic in wastewaters: A comprehensive review. Chemical Engineering Journal 317: 707-725.
- Thakur S, Chauhan M (2016) Removal of malachite green dye from aqueous solution by electrocoagulation with stainless steel electrodes. International Journal of Engineering Sciences & Research Technology 5(6): 515-521.
- Núñez J, Larral J, Roeckel M, Fernández K, Maril M, et al. (2023) Operational variable effect on the COD removal efficiency using electrocoagulation in landfill leachate treatment. Environmental Science: Water Research & Technology 9(3): 781-793.
- Orori O, Etiegni L, Senelwa K, Mwamburi M, Balozi K, et al. (2010) Electro-coagulation treatment efficiency of graphite, iron and aluminum electrodes using alum and wood ash electrolytes on a Kraft pulp and paper mill effluent. Water Science and Technology 62(7): 1526-1535.
- Akhter F, Soomro SA, Siddique M, Ahmed M (2021) Pollutant removal efficiency of electrocoagulation method from industrial wastewater: Comparison with other treatment methods and key operational parameters-a comparative study review. Water, Air, & Soil Pollution 232: 1-13.
- Tyagi N, Mathur S, Kumar D (2014) Electrocoagulation process for textile wastewater treatment in continuous up flow reactor. Journal of Scientific & Industrial Research 73: 195-198.
- Hashim KS, Ali SSM, AlRifaie JK, Kot P, Shaw A, et al. (2020) Escherichia coli inactivation using a hybrid ultrasonic-electrocoagulation reactor. Chemosphere 247: 125868.
- Gijzen H (2002) Anaerobic digestion for sustainable development: A natural approach. Water Science and Technology 45(10): 321-328.
- Janpoor F, Torabian A, Khatibikamal V (2011) Treatment of laundry wastewater by electrocoagulation. Journal of Chemical Technology & Biotechnology 86(8): 1113-1120.
- Grau P (1997) Integrated water and waste management. Water Science and Technology 33(8): 11- 20.
- Grau P (1996) Low-cost wastewater treatment. Water Science and Technology 33(8): 39-46.
- Asselin M, Drogui P, Brar SK, Benmoussa H, Blais JF (2008) Organics removal in oily bilgewater by electrocoagulation process. Journal of Hazardous Materials 151(2-3): 446-455.
- Amour A, Merzouk B, Leclerc JP, Lapicque F (2016) Removal of reactive textile dye from aqueous solutions by electrocoagulation in a continuous cell. Desalination and Water Treatment 57(48-49): 22764-22773.
- Chen G, Betterton E, Arnold R (1999) Electrolytic oxidation of trichloroethylene using a ceramic anode. Journal of Applied Electrochemistry 29: 961-970.
- Malakootian M, Yousefi N, Fatehizadeh A (2011) Survey efficiency of electrocoagulation on nitrate removal from aqueous solution. International Journal of Environmental Science & Technology 8: 107-114.
- Bazrafshan E, Moein H, Mostafapour FK, Nakhaie S (2013) Application of electrocoagulation process for dairy wastewater treatment. Journal of Chemistry 2013.
- Kabdaşlı I, Arslan-Alaton I, Ölmez-Hancı T, Tünay O (2012) Electrocoagulation applications for industrial wastewaters: A critical review. Environmental Technology Reviews 1(1): 2-45.
- Vidal J, Espinoza C, Contreras N, Salazar R (2017) Elimination of industrial textile dye by electrocoagulation using iron electrodes. Journal of the Chilean Chemical Society 62(2): 3519-3524.
- Gürses A, Yalçin M, Doğar C (2002) Electrocoagulation of some reactive dyes: A statistical investigation of some electrochemical variables. Waste Management 22(5): 491-499.
- Jing G, Ren S, Pooley S, Sun W, Kowalczuk PB, et al. (2021) Electrocoagulation for industrial wastewater treatment: An updated review. Environmental Science: Water Research & Technology 7(7): 1177-1196.
- Shivayogimath C, Watawati C (2013) Treatment of solid waste leachate by electrocoagulation technology. Int J Res Eng Technol 2: 266-269.
- Mahajan R, Khandegar V, Saroha AK (2013) Treatment of hospital operation theatre effluent by electrocoagulation. International Journal 4(2).
- Mahmad MKN, Rozainy MMR, Abustan I, Baharun N (2016) Electrocoagulation process by using aluminium and stainless-steel electrodes to treat total chromium, colour and turbidity. Procedia Chemistry 19: 681-686.
- Mahmoud MS, Farah JY, Farrag TE (2013) Enhanced removal of methylene blue by electrocoagulation using iron electrodes. Egyptian Journal of Petroleum 22(1): 211-216.
- Majlesi M, Mohseny SM, Sardar M, Gol Mohammadi S, Sheikh Mohammadi A (2016) Improvement of aqueous nitrate removal by using continuous electrocoagulation/electro flotation unit with vertical monopolar electrodes. Sustainable Environment Research 26(6): 287-290.
- Marol C, Puneet K, Prashant Y (2017) Treatment of dairy industry wastewater by Electrocoagulation (EC) technique removal of BOD, COD, turbidity and color. Int J Innov Res Sci Technol 4: 246-251.
- Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: A review. Journal of environmental management 92(3): 407-418.
- Gajjar NS, Patel MN (2013) Treatment of paint (Emulsion) industry wastewater by electrocoagulation. Journal of Environmental Science, Toxicology and Food Technology 3(5): 42-45.
- Espinoza-Quinones FR, Fornari MM, Modenes AN, Palacio SM, Trigueros DE, et al. (2009) Electrocoagulation efficiency of the tannery effluent treatment using aluminium electrodes. Water Science and Technology 60(8): 2173-2185.
- Essadki A, Bennajah M, Gourich B, Vial C, Azzi M, et al. (2008). Electrocoagulation/electro flotation in an external-loop airlift reactor-Application to the decolorization of textile dye wastewater: A case study. Chemical Engineering and Processing: Process Intensification 47(8): 1211-1223.
- Sarala C (2012) Domestic wastewater treatment by electrocoagulation with Fe-Fe electrodes. International Journal of Engineering Trends and Technology 3(4): 530-533.
- Drogui P, Benmoussa H, Asselin M, Blais JF, Brar SK (2013) Electrochemical removal of organics and oil from sawmill and ship effluents. Journal of Environmental Engineering and Science 36(3): 88-97.
- Meidinariasty A, Bow Y, Rusnadi I, Fuadi AL (2019) Treatment of leachate from garbage using electrocoagulation type MP-P (Mono Polar-Paralel) methode. Paper presented at the Journal of Physics 1167(1): 7.
- Sharma S, Can OT, Hammed M, Nawarathna D, Simsek H (2018) Organic pollutant removal from edible oil process wastewater using electrocoagulation. IOP Conference Series: Earth and Environmental Science 142: 10-12.
- Shen M, Zhang Y, Almatrafi E, Hu T, Zhou C, et al. (2022) Efficient removal of microplastics from wastewater by an electrocoagulation process. Chemical Engineering Journal 428: 131161.
- Kuokkanen V, Kuokkanen T (2013) Recent applications of electrocoagulation in treatment of water and wastewater: A review 3(2).
- Lin SH, Peng CF (1994) Treatment of textile wastewater by electrochemical method. Water research 28(2): 277-282.
- Karichappan T, Venkatachalam S, Jeganathan PM, Sengodan K (2013) Treatment of rice mill wastewater using continuous electrocoagulation technique: Optimization and modelling. Journal of the Korean Chemical Society 57(6): 761-768.
- Katal R, Pahlavanzadeh H (2011) Influence of different combinations of aluminum and iron electrode on electrocoagulation efficiency: Application to the treatment of paper mill wastewater. Desalination 265(1-3): 199-205.
- Dehghani M, Seresht SS, Hashemi H (2014) Treatment of hospital wastewater by electrocoagulation using aluminum and iron electrodes. International Journal of Environmental Health Engineering 3(1): 15.
- Perng YS, Bui HM (2014) Decolorization of Reactive Red 195 solution by electrocoagulation process. Journal of Vietnamese Environment 5(1): 22-26.
- Alizadeh M, Ghahramani E, Sadeghi S (2015) Removal of reactive green 19 dye from synthetic wastewater using electrocoagulation and aluminum electrodes. Journal of Advances in Environmental Health Research 3(1): 42-48.
- Alizadeh M, Ghahramani E, Zarrabi M, Hashemi S (2015) Efficient de-colorization of methylene blue by electro-coagulation method: Comparison of iron and aluminum electrode. Iranian Journal of Chemistry and Chemical Engineering 34(1): 39-47.
- Miranzadeh M, Rabbani D, Dehqan S (2012) Electrocoagulation process for removal of adenosine-5'-monophosphate and sodium hexametha phosphate from synthetic wastewater. International Journal of Physical Sciences 7(10): 1571-1577.
- Ghanbari F, Moradi M, Eslami A, Emamjomeh MM (2014) Electrocoagulation/flotation of textile wastewater with simultaneous application of aluminum and iron as anode. Environmental Processes 1: 447-457.
- Un UT, Ocal SE (2015) Removal of heavy metals (Cd, Cu, Ni) by electrocoagulation. International Journal of Environmental Science and Development 6(6): 425-429.
- Yang CH, Lee CC, Wen TC (2000) Hypochlorite generation on Ru-Pt binary oxide for treatment of dye wastewater. Journal of Applied Electrochemistry 30: 1043-1051.
- Yao J, Wang L, Zhang G, Tao J, Shi X, et al. (2023) Plate structure optimization and performance study of a new continuous flow electrocoagulation reactor. Chemical Engineering Research and Design 194: 693-704.
- Yari AR, Alizadeh M, Hashemi S, Biglari H (2013) Efficiency of electrocoagulation for removal of reactive yellow 14 from aqueous environments. Archives of Hygiene Sciences 2(1): 7-15.
- Getaye M, Hagos S, Alemu Y, Tamene Z, Yadav O (2017) Removal of malachite green from contaminated water using electro-coagulation technique. J Anal Pharm Res 6(4): 00184.
- Yu M, Jia J, Liu X, Cui J, Xi B, et al. (2019) P-Arsanilic acid degradation and arsenic immobilization by a disilicate-assisted iron/aluminum electrolysis process. Chemical Engineering Journal 368: 428-437.
- Gengec E, Kobya M, Demirbas E, Akyol A, Oktor K (2012) Optimization of baker's yeast wastewater using response surface methodology by electrocoagulation. Desalination 286: 200-209.
- Gautam P, Kumar S (2022) Reduction of chemical oxygen demand through electrocoagulation: An exclusive study for hazardous waste landfill leachate. Environmental Science and Pollution Research 29(5): 7583-7594.
- Zhang S, Zhang J, Wang W, Li F, Cheng X (2013) Removal of phosphate from landscape water using an electrocoagulation process powered directly by photovoltaic solar modules. Solar Energy Materials and Solar Cells 117: 73-80.
- Feng C, Sugiura N, Shimada S, Maekawa T (2003) Development of a high-performance electrochemical wastewater treatment system. Journal of Hazardous Materials 103(1-2): 65-78.
- El-Shazly AH, Daous M (2013) Kinetics and performance of phosphate removal from hot industrial effluents using a continuous flow electrocoagulation reactor. International Journal of Electrochemical Science 8(1): 184-194.
- Drogui P, Asselin M, Brar SK, Benmoussa H, Blais JF (2009) Electrochemical removal of organics and oil from sawmill and ship effluents. Canadian Journal of Civil Engineering 36(3): 529-539.
- Drogui P, Asselin M, Brar SK, Benmoussa H, Blais JF (2008) Electrochemical removal of pollutants from agro-industry wastewaters. Separation and Purification Technology 61(3): 301-310.
- Reátegui Romero W, Flores-Del Pino LV, Guerrero-Guevara JL, Castro-Torres J, et al. (2018) Benefits of electrocoagulation in treatment of wastewater: Removal of Fe and Mn metals, oil and grease and cod: Three case studies. Int J Appl Eng Res 13(8): 6450-6462.
- Dermentzis, K, Karakosta K, Kokkinos N, Mitkidou S, Stylianou M, et al. (2023) Photovoltaic-driven electrochemical remediation of drilling fluid wastewater with simultaneous hydrogen production. Waste Management & Research 41(1): 155-163.
- Rusdianasari R, Bow Y, Dewi T (2019) Peat water treatment by electrocoagulation using aluminium electrodes. IOP Conference Series, Indonesia, p. 258.
- Denier Gon HA, Gerlofs-Nijland ME, Gehrig R, Gustafsson M, Janssen N, et al. (2013) The policy relevance of wear emissions from road transport, now and in the future-an international workshop report and consensus statement. Journal of the Air & Waste Management Association 63(2): 136-149.
- Rusdianasari R, Taqwa A, Jaksen J, Syakdani A (2017) Treatment of landfill leachate by electrocoagulation using aluminum electrodes. Matec Web of Conferences, Bulgaria.
- Crittenden JC, Trussell RR, Hand DW, Howe KJ, Tchobanoglous G (2012) MWH's water treatment: Principles and design. (3rd edn), John Wiley & Sons, New Jersey, USA.
- Chou WL, Wang CT, Huang KY (2009) Effect of operating parameters on indium (III) ion removal by iron electrocoagulation and evaluation of specific energy consumption. Journal of Hazardous Materials 167(1-3): 467-474.
- Demirbas E, Kobya M (2017) Operating cost and treatment of metalworking fluid wastewater by chemical coagulation and electrocoagulation processes. Process Safety and Environmental Protection 105: 79-90.
- Chorghe D, Sari MA, Chellam S (2017) Boron removal from hydraulic fracturing wastewater by aluminum and iron coagulation: mechanisms and limitations. Water Research 126: 481-487.
- Chen X, Chen G, Yue PL (2000) Separation of pollutants from restaurant wastewater by electrocoagulation. Separation and Purification Technology 19(1-2): 65-76.
- Dash BP, Chaudhari S (2005) Electrochemical denitrification of simulated ground water. Water Research 39(17): 4065-4072.
- Daneshvar N, Khataee A, Ghadim AA, Rasoulifard M (2007) Decolorization of CI acid yellow 23 solution by electrocoagulation process: Investigation of operational parameters and evaluation of Specific Electrical Energy Consumption (SEEC). Journal of Hazardous Materials 148(3): 566-572.
- Bruguera Casamada C, Sirés I, Brillas E, Araujo RM (2017) Effect of electrogenerated hydroxyl radicals, active chlorine and organic matter on the electrochemical inactivation of Pseudomonas aeruginosa using BDD and dimensionally stable anodes. Separation and Purification Technology 178: 224-231.
- Bratby J (2016) Coagulation and flocculation in water and wastewater treatment. (3rd edn), IWA Publishing, London, UK.
- Bonfatti F, Ferro S, Lavezzo F, Malacarne M, Lodi G, et al. (2000) Electrochemical incineration of glucose as a model organic substrate II Role of active chlorine mediation. Journal of the Electrochemical Society 147(2): 592.
- Can O (2014) COD removal from fruit-juice production wastewater by electrooxidation electrocoagulation and electro-Fenton processes. Desalination and Water Treatment 52(1-3): 65-73.
- Biswas B, Goel S (2022) Electrocoagulation and electrooxidation technologies for pesticide removal from water or wastewater: A review. Chemosphere 302: 134709.
- Bhagawan D, Poodari S, Golla S, Himabindu V, Vidyavathi S (2016) Treatment of the petroleum refinery wastewater using combined electrochemical methods. Desalination and Water Treatment 57(8): 3387-3394.
- Behbahani M, Moghaddam MR, Arami M (2013) Phosphate removal by electrocoagulation process: Optimization by response surface methodology method. Environmental Engineering & Management Journal 12(12): 2397-2405.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






