- Submissions

Full Text
Examines in Marine Biology & Oceanography
Spirulina platensis, A Functional Feed Additive in Finfish Aquaculture – An Update
Einar Ringø*
Norwegian College of Fishery Science, Faculty of Bioscience, Fisheries and Economics, UiT The Arctic University of Norway, Norway
*Corresponding author: Einar Ringø, Norwegian College of Fishery Science, Faculty of Bioscience, Fisheries and Economics, UiT The Arctic University of Norway, Tromsø, Norway
Submission: September 19, 2024;Published: September 25, 2024

ISSN 2578-031X Volume7 Issue2
Opinion
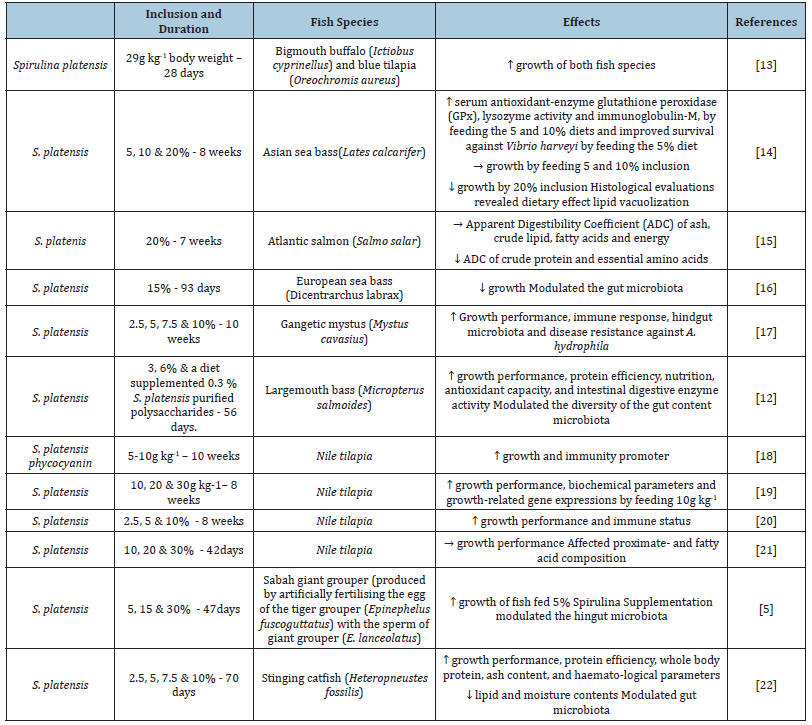
As the price of fish meal is high, cheaper suitable alternatives are evaluated. Spirulina could be a functional alternative protein- and vitamins source in finfish aquaculture. The blue-green algae Spirulina, microalgae, are multicellular filamentous cyanobacteria [1] and grows well at pH around 8.5 and above, and at temperature around 30 °C. Analysis have shown high amounts of antioxidants, microelements, vitamins, essential amino acids, y-linoleic acid and phenolic compounds [2,3]. In addition, Spirulina has revealed cholesterol-lowering properties, beneficial effects in various inflammatory diseases [4], and modulated the gut microbiota composition [5,6]. Due to its high protein content, 60-70% of dry weight [7], Spirulina is one of the most used microalgae in aquaculture as feed supplements to replace fish meal to a lower coast and to improve the health and growth performance of aquatic animals [8-10]. In addition, the microalgae are also utilized for treating aquaculture wastewater and reducing environmental impact [11,12]. The present study, address to present and update overview on Spirulina platensis administration to finfish, and Table 1 shows beneficial effects in finfish the last five years. The first study on the effect of Spirulina platensis, on fish was reported by Stanley and Jones [13]. Aquarium held bigmouth buffalo (Ictiobus cyprinellus) were fed the blue-green algae at a daily rate of 29g dry weight kg-1 body weight for 28 days, and a growth rate of 14g kg-1 and a food conversion rate of 2.0 was recorded. Similar results were revealed by feeding blue tilapia (Oreochromis aureus).
Asian sea bass (Lates calcarifer)
In a study with Asian sea bass, Siddik et al. [14] fed the fish 5 and 10% inclusion and showed similar growth as control fish, no inclusion of Spirulina, in contrast to 20% which revealed significant lower growth. Most parameters investigated were not affected by Spirulina administration, but serum antioxidant-enzyme glutathione peroxidase (GPx) and immunological indices, lysozyme activity and immunoglobulin-M, were significantly higher by feeding the 5 and 10% diets vs. fish fed the control diet. In addition, histological evaluations revealed no aberrant hepatocytes in the livers of any of the dietary groups, except for the 20% diet, and in this feeding group higher lipid vacuolization when compared to the other dietary groups. An important finding was improved survival of fish fed 5% following exposure to Vibrio harveyi.
Atlantic salmon (Salmo salar)
Tibbetts et al. [15] revealed in a seven week study with Atlantic salmon fed a reference diet consisting of FM, poultry by-product meal, wheat gluten meal, soy protein concentrate and corn protein concentrate and a diet added 20% Spirulina that Apparent Digestibility Coefficients (ADCs) of ash, crude lipid and fatty acids and energy by Spirulina feeding were not affected, in contrast to crude protein ADC and essential amino acids was slightly reduced.
Table 1:Beneficial effects of Spirulina platensis in finfish, since 2020.
Source: ↑ - positive effect; → - no. significant effect; ↓ - decrease effect.
European sea bass (Dicentrarchus labrax)
Pérez-Pascual et al. [16] revealed that 15% inclusion of S. platensis fed to European sea bass negatively affected growth and modulated the OTUs richness, and Shannon index of the gut content microbiota. As the gut microbiota is an important factor affecting fish health, the autochthonous gut microbiota should be investigated and not only the bacterial composition in gut content.
Gangetic mystus (Mystus cavasius)
Mamun et al. [17] showed that when more than 7.5% of FM was replaced by S. platensis meal, body weight, specific growth rate, protein efficiency and feed conversion ratio were improved compared to gangetic mystus fed the control diet. The protein efficiency and feed conversion ratio were also positively affected by dietary S. platensis meal supplementation. The replacement of 7.5–10% fishmeal with S. platensis red- and white blood cells, hemoglobin, hematocrit, and mean corpuscular hemoglobin levels rose. Serum lysozyme value increased with increasing dietary S. platensissupplementation. Bacterial analysis showed that the autochthonous hindgut microbiota of hindgut was affected, an increase in the relative abundance of Pseudomonas, Lentibacillus, and Lactobacillus by increasing supplementation of S. platensis. Improved disease resistance against A. hydrophila was noticed at 10% inclusion.
Largemouth bass (Micropterus salmoides). Zhang et al. [12] fed juvenile largemouth bass, diets containing 0 (no inclusion, control), 3 (SP3), 6% S. platensis (SP6) and a diet supplemented with 0.3% S. platensis Purified Polysaccharides (PSP) for 56 days. Administration of SP3 and SP6 displayed positive results as the diets significantly improved growth performance, lower hepatosomatic indices, increased whole-body crude protein, protein efficiency and muscle amino acid composition vs. control fed fish. However, crude lipid content decreased by feeding the SP diets. Liver superoxide dismutase activity increased significantly, while malondialdehyde levels declined. Levels of serum triglyceride significantly decreased by feeding 3% Spirulina than in the control. Low-density lipoprotein cholesterol decreased significantly in the SP3 and SP6 dietary groups, but no significant effect was noticed in the PSP group. Intestinal enzymes activities were affected by Spirulina administration, as trypsin and amylase activities significantly enhanced in the SP3, and SP6 and PSP groups, while lipase activities in SP 3 and PSP groups increased significantly compared to the control group. Analysis of the intestinal content microbiota analysis showed modulation as significantly higher Shannon index scores were noticed when fish were fed diets added S. platenis compared to control fed fish.
Nile tilapia (Oreochromis niloticus). El-Araby et al. [18] conducted a 10-weeks feeding trial with Nile tilapia to determine the effect of S. platensis phycocyanin (SPC) and revealed that supplementation enhanced growth performance, most intestinal morphometric measures, serum catalase and superoxide dismutase activity and immune response indicators (interleukin 10 (IL10), lysozyme activity, complement 3, and IgM serum levels. In contrast, reduced glutathione level and decreased malondialdehyde level was noticed with SPC supplementation. Nile tilapia fed diet supplemented with 10g S. platensis meal kg-1 enhanced growth, feed utilization, serum protein, globulin levels and growth hormone expression in the brain and muscle compared the other treatments, control and 20 and 30g inclusion [19]. Youssef et al. [20] concluded that supplementation of S. platensis to Nile tilapia diets increased body weight and length, weight gain, FCR, phagocytic activity, intestinal parameters as well as gut histology.
In recent study, Nile tilapia was fed diets added 10, 20 and 30% S. platensis and displayed no significant effect on growth performance [21]. Analysis of proximate composition showed significantly higher protein content by feeding fish the highest inclusion level, while lipid content was highest by feeding fish 20% Spirulina. Whole fatty acid composition showed difference between the treatments, as total saturated fatty acids and oleic acid, increased significantly in the fish fed the Spirulina diets. In contrast, linoleic acid was significantly higher in fish fed commercial diet. Of the omega-3 fatty acids, it was noticed that linolenic-, eicosapentaenoic-, and docosahexaenoic acid were significantly higher in fish fed 30% S. platensis.
Sabah giant grouper (Epinephelus fuscoguttatus and E. lanceolatus). Man et al. [5] reported that grouper fed a 5% Spirulinabased diet almost tripled its the total weight gain compared to control fed fish. Microbial communities in the hindgut of fish revealed that Simpson’s diversity index showed high bacterial diversities in fish fed the Spirulina diets. At genus level, Tolumonas displayed the highest relative abundance in the hindgut of control fish, in contrast to genus Vibrio showing the highest relative abundance in fish fed the Spirulina diets. Stinging catfish (Heteropneustes fossilis). In a study conducted by Zahan et al. [22] the authors determined the inclusion effect of S. platensis (2.5, 5, 7.5 and 10%) as replacing fish meal in diets to stinging catfish for 70 days. Significantly improved growth performance and serum lysozyme activity was recorded by feeding the fish SP7.5 and SP10 diets vs. control. An important finding of the present study was that challenge with Aeromonas hydrophila for 15 days displayed improved survival, 70% in fish fed the SP7.5 diet compared to the control, 30%. Dietary S. platensis inclusion modulated the autochthonous gut microbiota by increasing the relative abundance Pseudomonas, Lactobacillus and Lentibacillus. The relative abundance of the gut microbiota by feeding SP5, Pseudomonas (28%) and Lactobacillus (~19%) were more prevalent, in contrast to Lentibacillus (~29%) in SP2.5, while Corynebacterium (18%) was the dominant genera by control feeding.
Nile tilapia (Oreochromis niloticus). El-Araby et al. [18] conducted a 10-weeks feeding trial with Nile tilapia to determine the effect of S. platensis phycocyanin (SPC) and revealed that supplementation enhanced growth performance, most intestinal morphometric measures, serum catalase and superoxide dismutase activity and immune response indicators (interleukin 10 (IL10), lysozyme activity, complement 3, and IgM serum levels. In contrast, reduced glutathione level and decreased malondialdehyde level was noticed with SPC supplementation. Nile tilapia fed diet supplemented with 10g S. platensis meal kg -1 enhanced growth, feed utilization, serum protein, globulin levels and growth hormone expression in the brain and muscle compared the other treatments, control and 20 and 30g inclusion [19]. Youssef et al. [20] concluded that supplementation of S. platensis to Nile tilapia diets increased body weight and length, weight gain, FCR, phagocytic activity, intestinal parameters as well as gut histology.
In recent study, Nile tilapia was fed diets added 10, 20 and 30% S. platensis and displayed no significant effect on growth performance [21]. Analysis of proximate composition showed significantly higher protein content by feeding fish the highest inclusion level, while lipid content was highest by feeding fish 20% Spirulina. Whole fatty acid composition showed difference between the treatments, as total saturated fatty acids and oleic acid, increased significantly in the fish fed the Spirulina diets. In contrast, linoleic acid was significantly higher in fish fed commercial diet. Of the omega-3 fatty acids, it was noticed that linolenic-, eicosapentaenoic-, and docosahexaenoic acid were significantly higher in fish fed 30% S. platensis.
Sabah giant grouper (Epinephelus fuscoguttatus and E. lanceolatus). Man et al. [5] reported that grouper fed a 5% Spirulinabased diet almost tripled its the total weight gain compared to control fed fish. Microbial communities in the hindgut of fish revealed that Simpson’s diversity index showed high bacterial diversities in fish fed the Spirulina diets. At genus level, Tolumonas displayed the highest relative abundance in the hindgut of control fish, in contrast to genus Vibrio showing the highest relative abundance in fish fed the Spirulina diets. Stinging catfish (Heteropneustes fossilis). In a study conducted by Zahan et al. [22] the authors determined the inclusion effect of S.platensis (2.5, 5, 7.5 and 10%) as replacing fish meal in diets to stinging catfish for 70 days. Significantly improved growth performance and serum lysozyme activity was recorded by feeding the fish SP7.5 and SP10 diets vs. control. An important finding of the present study was that challenge with Aeromonas hydrophila for 15 days displayed improved survival, 70% in fish fed the SP7.5 diet compared to the control, 30%. Dietary S. platensis inclusion modulated the autochthonous gut microbiota by increasing the relative abundance Pseudomonas, Lactobacillus and Lentibacillus. The relative abundance of the gut microbiota by feeding SP5, Pseudomonas (28%) and Lactobacillus (~19%) were more prevalent, in contrast to Lentibacillus (~29%) in SP2.5, while Corynebacterium (18%) was the dominant genera by control feeding.
Conclusion
Spirulina could be a functional alternative protein- and vitamins source in finfish aquaculture, as supplementation of Spirulina reveal growth enhancement, enhance cellular and humoral immunities, modulate the gut microbiota and disease resistance towards pathogenic infection of various fish species. In addition, Spirulina can be used for treating wastewater. Even though beneficial properties are revealed, more research is needed to clarify the beneficial effects of Spirulina platensis in challenge studies of aquatic organisms.
References
- Ciferri O (1983) Spirulina, the edible microorganism. Microbiology Reviews 47(4): 551-578.
- Ku CS, Yang Y, Park Y, Lee J (2013) Health benefits of blue-green algae: Prevention of cardiovascular disease and nonalcoholic fatty liver disease. Journal of Medical Food 16(2): 103-111.
- Serban MC, Sahebkar A, Dragan S, Stoichescu-Hogea G, Ursoniu S, et al. (2016) A systematic review and meta-analysis of the impact of spirulina supplementation on plasma lipid concentrations. Clinical Nutrition 35(4): 842-851.
- Kumar R, Hegde AS, Sharma K, Parmar P, Srivatsan V (2022) Microalgae as a sustainable source of edible proteins and bioactive peptides – Current trends and future prospects. Food Research International 57: 111338.
- Man YB, Zhang F, Ma KL, Mo WY, Kwan HS, et al. (2020) Growth and intestinal microbiota of Sabah giant grouper reared on food waste-based pellets supplemented with spirulina as a growth promoter and alternative protein source. Aquaculture Reports 18: 100553.
- Brito Alves JL, Costa PCT, Sales LCS, Siva Luis CC, Teixeira Bezerra TP, et al. (2024) Shedding light on the impacts of Spirulina platensis on gut microbiota and related health benefits. Critical Reviews in Food Science and Nutrition 29: 1-14.
- Deng R, Chow TJ (2010) Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae spirulina. Cardiovascular Therapeutics 28(4): e33-e45.
- Li L, Liu H, Zhang P (2022) Effect of Spirulina meal supplementation on growth performance and feed utilization in fish and shrimp: A meta-analysis. Aquaculture Nutrition 2022: 8517733.
- Trevi S, Webster U, Consuegra S, Leaniz CG (2023) Benefits of the microalgae Spirulina and Schizochytrium in fish nutrition: A meta-analysis. Scientific Reports 13(1): 2208.
- Rana M, Mandal S, Kabita S (2024) Spirulina in fish immunity development: Find the black box. Reviews in Fish Biology and Fisheries 34: 623-646.
- Wuang SC, Khin MC, Chua PQD, Luo YD (2016) Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Research 15: 59-64.
- Zhang F, Man YB, Mo WY, Wong MH (2020) Application of Spirulina in aquaculture: A review on wastewater treatment and fish growth. Reviews in Aquaculture 12(2): 582-599.
- Stanley JG, Jones JB (1976) Feeding algae to fish. Aquaculture 7(3): 219-223.
- Siddik MAB, Vatsos JN, Rahman MdA, Duc Pham H (2022) Selenium-enriched Spirulina (SeE-SP) enhance antioxidant response, immunity and disease resistance in juvenile Asian seabass, Lates calcarifer. Antioxidants 11(8): 1572.
- Tibbetts SM, MacPherson MJ, Park KC, Melanson RJ, Patelakis SJJ (2023) Composition and apparent digestibility coefficients of essential nutrients and energy of cyanobacterium meal produced from Spirulina (Arthrospira platensis) for freshwater-phase Atlantic salmon (Salmo salar L.) pre-smolt. Algal Research 70: 103017.
- Pérez-Pascual D, Estelle J, Dutto G, Rodde C, Bernardet J-F, et al. (2020) Growth performance and adaptability of European sea bass (Dicentrarchus labrax) gut microbiota to alternative diets free of fish products. Microorganisms 8(9): 1346.
- Mamun MdA, Hossain MdA, Saha J, Khan S, Akter T, et al. (2023) Effects of spirulina Spirulina platensis as feed additive on growth performance and immunological response of Gangetic mystus Mystus cavassius. Aquaculture Reports 30: 101553.
- El-Araby DA, Amer SA, Attia GA, Osman A, Fahmy, EM, et al. (2022) Dietary Spirulina platensis phycocyanin improves growth, tissue histoarchitecture, and immune responses, with modulating immunoexpression of CD3 and CD20 in Nile tilapia, Oreochromis niloticus. Aquaculture 546: 737413.
- Abozaid H, Elnady ASM, Aboelhassan BM, Mansour H, Abedo AE, et al. (2023) Impact of Spirulina as dietary supplement on growth performance, blood biochemical parameters, and expression of growth-related genes in Nile tilapia (Oreochromis niloticus). Egyptian Journal of Veterinary Science 54(6): 1265-1277.
- Youssef IM, Saleh ESE, Tawfeek SS, Abdel-Fadeel AAA, Abdel-Razik A-RH, et al. (2023) Effects of Spirulina platensis on growth, hematological, biochemical, and immunological parameters of Nile tilapia (Oreochromis niloticus). Tropic Animal Health and Production 55(4): 275.
- Soma K, Kals J, Opiyo MA, Ndambi A, García-Cubero R, et al. (2024) Toward sustainable food systems: can spirulina (Arthrospira platensis) become a sustainable source of protein to enhance the nutritional benefits of cultured Nile tilapia (Oreochromis niloticus)? Frontiers in Sustainable Food Systems 8: 283150.
- Zahan N, Hossain MA, Islam MR, Saha J, Akter T, et al. (2024) Effects of dietary Spirulina platensis on growth performance, body composition, haematology, immune response, and gut microflora of stinging catfish Heteropneustes fossilis. Aquaculture Reports 35: 101997.
© 2024 Einar Ringø. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






