- Submissions

Full Text
Examines in Marine Biology & Oceanography
A New Way for Discovery of Sponge-Associated Bacteria with Biotechnological Potential
Alessia Lunetta, Simone Cappello*, Antonietta Specchiulli, Tommaso Scirocco, Sabrina Patania and Maria Genovese
Institute for Biological Resources and Marine Biotechnology (Irbin)-Seine, Section of Messina, Italy
*Corresponding author: Simone Cappello, Institute for Biological Resources and Marine Biotechnology (Irbin)-Seine, Section of Messina, Sp. San Raineri, 86 - 98122 Messina, Italy
Submission: January 25, 2024;Published: February 14, 2024

ISSN 2578-031X Volume6 Issue4
Opinion
Sponges (Phylum Porifera) are host animals for various symbiotic microorganisms. The ecological value of the sponges together with the symbiotic association with bacteria, make them an ideal system for biotechnological studies. Sponges are filter organisms, the presence of numerous small pores on their surface allow the entry of water and the microorganisms on which they feed. Microorganisms capable of resisting the sponges’ digestive process and immune response become symbionts of the same [1]. Sponges pump up to 24 m3 kg-1 day-1 of seawater that contains 1-5×106 bacteria ml-1, thus making up to 40% of their total biomass [2] and exceeding by two to three orders of magnitude the number of bacteria in seawater [3]. The abundance of sponge-associated bacteria appears to be proportional to the capacity of the sponge’s irrigation system; infact sponges with an inadequate irrigation system contain few numbers of bacteria than sponges with a well adequate irrigation system. Sponges can distinguish between symbiotic microorganisms, pathogens and food-borne bacteria. The bacteria associated with sponges may be species-specific bacteria or be naturally influenced by the environment in which they are found. Not only do sponges host diverse microbial communities, but most are metabolically active within their respective hosts (Kamke et al. 2010); in fact, studies have shown that the production of some metabolites initially mistakenly attributed to sponges are actually biosynthesized by the symbionts themselves. Consequently, culturing the micro-organism itself can provide a better source of the bioactive compound [4].
Over the years, therefore, interest in the study and cultivation of bacterial strains associated with sponges has increased; sponges from Arctic, temperate, tropical and subtropical regions have been studied, combining culture-dependent and culture-independent techniques [5,6] and using different culture media. Unlike other studies conducted to better understand the symbioses between marine microorganisms and macroorganisms, very little research has been conducted on the study of the resilience of the sponge-bacteria system in relation to climate change or other stress factors of anthropogenic origin.
The environmental contaminants (e.g., hydrocarbons and/or heavy metals) originating from human activities (e.g., industries, disposal plants, sewage treatment plants, shipbuilding areas) present themselves as very important forcing for the selection of micro-organisms capable of resisting and biodegrading (at various levels of efficiency) such substances. It has been amply proven how the presence of hydrocarbons in the marine environment can promote the selection of hydrocarbon-degrading and/or Hydrocarbon Obligate Bacteria (HOCB) that present themselves as decisive “actors” in environmental recovery processes [7].
HOCBs are bacteria exclusively belonging to the marine environment, the obligatory term indicates the metabolic peculiarity of degrading and using exclusively hydrocarbons as the sole source of carbon and energy. To this group belong different genera Alcanivorax, Oleispira, Thalassolitus, Oleiphilus, Cyclocalasticus, isolated in geographically distant parts of the world with different chemical and physical characteristics thus demonstrating how they are able to adapt to different environmental parameters. Current studies have shown that in the presence of a hydrocarbon pollution event, HOCB populations that were initially undetectable or present at low levels begin to proliferate, representing 90% of the entire microbial population present. An example is represented by Alcanivorax borkumensis, which represents a dominant bacterium in the presence of oil spills and in this regard its capacity for aerobic degradation of n-alkanes has been widely studied. The biodegradation process of aerobic n-alkanes is controlled by the multicomponent enzyme system, encoded by alkane hydroxylase genes (alk-B family), thus playing a key role in the bioremediation of marine environments contaminated by hydrocarbons. The alk-B system consisting of the alK-B1 and alk-B2 genes are simultaneously induced by n-alkanes and specifically involved in the oxidation of short- and medium-chain alkanes. More specifically, the biodegradation of n-alkanes is mediated through the oxidation of a terminal methyl group to a primary alcohol, then oxidized to an aldehyde and dehydrogenated to a fatty acid, and finally converted to acetyl-CoA via beta-oxidation [8].
Likewise, most studies have focused on a limited number of organisms (sponges). This has extremely reduced the possibility of bacterial isolates that depend only on different culture approaches in the laboratory. In this context, at the Institute for Biological Resources and Marine Biotechnologies (IRBIM) of the National Research Council (CNR) in Messina, we are conducting studies on the sponge Raspaciona aculeata (Johnston 1842) and its symbiotic bacteria. The study focuses on two parallel fronts: An ecological front and an applied biotechnological front. The ecological study understood in terms of biodiversity and abundance of associated bacterial communities. The applied biotechnological study is instead identified in the isolation, characterization and definition of the biotechnological potential of associated symbiotic bacteria collected in environments contaminated by chemical agents such as oil and its derivatives (Figure 1).
Figure 1:Relationship between the marine sponge Raspaciona aculeata and hydrocarbonclastic and/or hydrocarbon-degrading bacteria in relation to possible sources of environmental contamination by hydrocarbons and the operational and functional strategies of the application of symbiotic bacteria.
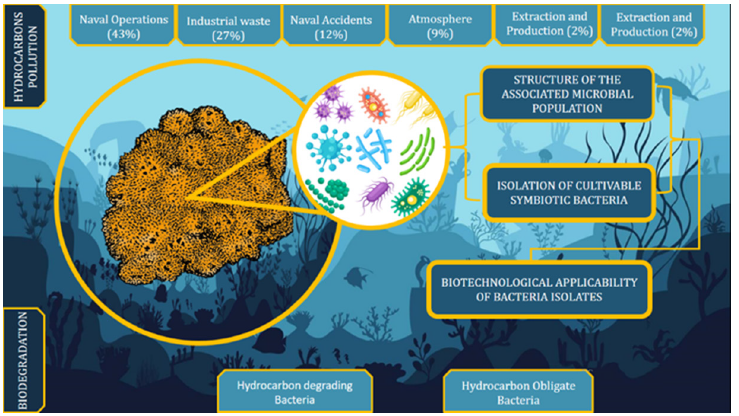
Preliminary data from our studies conducted on sponge samples collected in port areas chronically contaminated by oily waste originating from maritime transport have allowed us to isolate bacteria associated with the genus Alcanivorax sp. Likewise, from the same type of samples, other bacterial strains are isolated as excellent producers of biosurfacts and bioplastics, highlighting their great bio applicative importance. In this context it is therefore necessary to continue research that aims not only to deepen the relationship between sponges and bacteria, but also the strategies they implement to cope with continuous and uncontainable environmental changes. A study on the improvement of bacterial cultivation techniques will contribute to further exploration of their possible biotechnological applications.
References
- Wilkinson CR (1987) Significance of microbial symbionts in sponge evolution and ecology. Symbiosis 4: 135-146.
- Vacelet J (1975) Electron microscopy study of the association between bacteria and sponges of the genus Verongia (Dictyoceratida). J. Microsc Biol Cell 23: 271-288.
- Webster NS, Hill RT (2001) The culturable microbial community of the great barrier reef sponge Rhopaloeides odorabile is dominated by an α-Proteobacterium. Marine Biology 138: 843-851.
- Schmidt EW, Obraztsova AY, Davidson SK, Faulkner DJ, Haygood MG (2000) Identification of the antifungal peptide-containing symbiont of the marine sponge Theonella swinhoei as a novel δ-proteobacterium, “Candidatus Entothe-onella palauensis". Mar Biol 136: 969-977.
- Sun W, Dai S, Jiang S, Wang G, Liu G, et al. (2010) Culture-dependent and culture-independent diversity of Actinobacteria associated with the marine sponge Hymeniacidon perleve from the South China Sea. Antonie Van Leeuwenhoek 98(1): 65-75.
- Kuo J, Yang Y, Lu MC, Wong TY, Sung PJ, et al. (2019) Antimicrobial activity and diversity of bacteria associated with Taiwanese marine sponge Theonella swinhoei. Ann Microbiol 69: 253-265.
- Cappello S, Caruso G, Zampino, D, Monticelli LS, Maimone G, et al. (2007) Microbial community dynamics during assays of harbour oil spill bioremediation: A microscale simulation study. Journal of Applied Microbiology 102(1): 184-194.
- Schneiker S, Dos Santos VAM, Bartels D, Bekel T, Brecht M, et al. (2006) Genome sequence of the ubiquitous hydrocarbon-degrading marine bacterium Alcanivorax borkumensis. Nature Biotechnology 24(8): 997-1004.
© 2024 Simone Cappello. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






