- Submissions

Full Text
Examines in Marine Biology & Oceanography
A Perspective on Emerging and Promising Aquatic Lower Eukaryotes as Novel Model Organisms for Cell Biology
Enrico Bracco1,2*
1Department of Oncology, Medical School, University of Torino, Italy
2Istituto Nazionale di Ricerca Metrologica, Italy
*Corresponding author: Enrico Bracco, Department of Oncology, Medical School, University of Torino, and Istituto Nazionale di Ricerca Metrologica, Italy, Email: enrico.bracco@unito.it
Submission: August 01, 2023;Published: August 10, 2023

ISSN 2578-031X Volume6 Issue2
Abstract
Choosing the most convenient model organism, to unravel biological and physiological questions, is an issue whose importance was already recognized at the beginning of the nineteenth century. Over the last decades, we learned that in several cases, the selection of a particular organism was serendipitous rather than scrupulously planned, and more unexpectedly some of their advantageous features were only subsequently unveiled. The usage of aquatic model organisms, starting from the unicellular Acetabularia and then later with Volvox and up more recently with choanoflagellates (i.e., Monosiga, Salpingoeca) has significantly contributed to dissecting many pivotal questions in the field of cell biology ranging from finding a functional role to the nucleus up to surveying the evolution of multicellularity. In the recent past, we have witnessed their genome sequencing and more recently the possibility to transfect many unicellular aquatic organisms -protists- letting to surmise their usage as novel potential model organisms to explore and scrutinize unaddressed biological issues.
Keywords:Eukaryotes; Cell biology; Nucleus; Biological issues; Developmental biology; Pathogen
Introduction
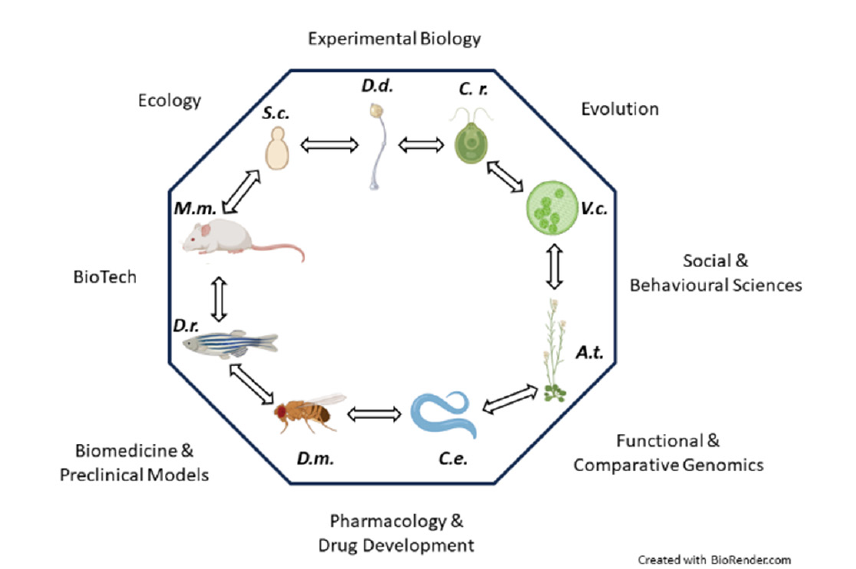
The current knowledge of the molecular mechanisms driving and sustaining cellular functions is mostly based on studies with model organisms established over the last century [1]. Regardless of being uni- or multi-cellular, it is generally considered a model organism, any organism of non-human species displaying inherently convenient features for tackling questions across the cell and developmental biology, as well as biomedicine, evolution, and behavioral sciences (Figure 1). Basically, a model organism should represent a larger group of organisms beyond itself and serve to explore different processes that should be shared across different taxa [2]. An ideal model organism possesses traits favoring its domestication and adaptation to the laboratory environment. Additionally, it should be easily cultivated/bred under controlled laboratory conditions, with a small size and a short generation time, and ultimately be genetically tractable [3]. Over the years the list of eukaryotic model organisms has grown enormously. Among the different model organisms, the protists, lower unicellular eukaryotic organisms that are not animal, plant, or fungus, display many of the model systems’ compelling features [4]. Furthermore, protists represent the predominating biomass hosted in the oceans and are major contributors to that of the terrestrial environment [5].
Figure 1:Model organisms are tools to unravel different questions ranging from basic research to biomedicine and social sciences. Schematics of some of the most representative model organisms are shown in the inner core of the octagonal shape (S.c.: Saccharomyces cerevisiae; D.d.: Dictyostelium discoideum; C.r.: Chlamydomonas reinhardtii; V.c.: Volvox carteri; A.t.: Arabidopsis thaliana; C.e.: Caenorhabditis elegans; D.m.: Drosophila melanogaster; M.m.: Mus musculus). Double-headed arrows symbolize collaborative communication among the different model organisms and their communities. Different scientific fields benefitting from model organisms research are depicted aside from the edges of the polygon.
A Short Glance at a Few Eukaryotes Model Organisms
Due to their specific properties, model organisms might exhibit their strongest impacts in rather different areas of biology. Among those that significantly contributed to expanding cell biology knowledge, and finding a role for an intracellular compartment, whose function was so far unknown, the marine unicellular alga Acetabularia mediterranea (or A. acetabulum) -J.V. Lamour, 1812- deserves a distinctive place. In those early days, by means of simple experiments involving the separation of the unicellular alga into nucleate and anucleate parts, enabled the collection of compelling evidence leading to suggest a role for the nucleus, namely, hosting the blueprint of the species [6,7]. Later, the multicellular alga Volvox carteri -F.R. Stein, 1878- has been broadly exploited as a model to investigate multicellularity and to untangle its role during evolution. Its hallmarks of complex multicellularity (e.g., asymmetric cell divisions, dimorphic sexes, coordinated tissuelevel morphogenesis, etc…) enabled to characterize the molecular basis of multicellularity, including genetic control of cell number, cell adhesion molecules, acquisition of organismal polarity, morphogenetic shape changes and conversion of the cell wall into the extracellular matrix, which are features shared with mammals. Unambiguously, the fruit fly Drosophila melanogaster -J.W. Meigen, 1830- has proven to be an excellent system to carry out genetic screening, and it is currently used to unravel the underlying mechanisms of morphogenesis and nervous system development. Interestingly, by taking advantage of fruit flies at the larval stage, it has been recently mapped the first entire connectome from the brain [8].
Nonetheless, an excellent model organism does not need to be multi-cellular as it occurs for yeasts (Saccharomyces cerevisiae -F.J.F. Meyen and E.C. Hansen, 1883- and Schizosaccharomyces pombe -U. Leupold, 1946-). Yeasts have been pioneers for decades in investigating very basic cell biology issues that have been later implied in causing and sustaining different human diseases. Furthermore, they represent an outstanding model to identify and characterize the molecular players controlling the cell cycle, as well as intracellular vesicular trafficking and protein interaction network [9]. Unique features are displayed by the social amoebae. The latter, whose most popular representative is Dictyostelium discoideum -K.B. Raper, 1934-, usually live as unicellular organisms but upon food depletion, they cease proliferating and gather to form a multicellular structure undergoing morphogenetic changes and differentiation [10]. D. discoideum is recognized as leading model to unravel the molecular mechanisms driving cell motility, including chemotaxis alongside other motility-linked processes such as cytokinesis and phagocytosis [11-13]. More recently, it has emerged as a powerful simple model for social evolution and for host-pathogen interaction and microbial infection [14].
Emerging Promising Aquatic Protists Model Organisms
Though marine unicellular lower eukaryote, protists, play major roles in the marine ecosystems, and occupy key positions in the tree of life, our knowledge of their cell biology remain scant. Currently, a handful of terrestrial and freshwater protists are considered model organisms including the amoebozoan Dictyostelium, the chlorophyte Chlamydomonas -G.M. Smith, 1945- and the ciliates Paramecium -O.F. Mull, 1773- and Tetrahymena -A. Lwoff, 1923- but there are no marine protists. This is a major drawback in our understanding of how they accomplish their functions, and in exploiting them to attain new insights into cell biology.
Among the various field of science, including cell biology, to increase our knowledge two main strategies are commonly foreseen: i) increasing the depth and ii) breadth. While going deeper into well-established biological model/s (i.e., yeast, fly, etc.,) is often achieved by technical innovations, the attaining of a broader view on long-studied processes is achieved by exploring the biological diversity. In this regards, marine protists have, recently, offered great inspiration. For instance, thanks to the genome sequencing of some Choanoflagellates (i.e., Monosiga brevicolis -Kent, 1880-) [15], which are considered the closest living organisms to animals, were identified several gene families formerly considered to be exclusively animal-specific, including Notch and Semaphorin/Plexins signalling pathways [15,16]. Furthermore, among Choanoflagellates the Salpingoeca rosetta -Dayel, 2011-has been recently recognized as a suitable model to investigate processes that are considered important from the physiological and pathological point of view in human (i.e., oxygen sensing and directional migration toward it, namely aerotaxis) [17]. Additionally, Choanoflagellates, possess an extraordinarily kinome repertoire [18]. Conversely, the marine protist Creolimax fragrantissima -W.L. Marshal, 2008- has shown to harbour a very low complexity kinome, but with unique feature that is a single cytoplasmic tyrosine kinase homolog of c-Src. Intriguingly, the C. fragrantissima Src -CfrSrc- primary structure displays a notable difference when compared with those of mammalian Src and receptor tyrosine kinases family members. At the level of the gatekeeper residue, in the active site cleft, CfrSrc harbours a leucine instead of a threonine residue that, on the contrary, characterize mammals’ tyrosine kinases [19]. Interestingly, clinically relevant tyrosine kinases mutations within the gatekeeper increases the affinity for ATP, as it occurs for EGFR in patients with advanced non-small cell lung cancer, thus contributing to drug resistance (i.e., tyrosine kinase inhibitors) [20].
Eventually, the enzymatic activity of CfrSrc is under the control of a phosphatase instead of being under the control of the kinase Csk, as it occurs for mammals. Overall, C. fragrantissima emerges as peculiar model to investigate the adaptation of tyrosine kinase signalling and regulatory mechanisms, as well as potential tool for high-throughput screening inquiry aimed to identify new tyrosine kinase inhibitors. Among the marine protists, the dinoflagellates (i.e., Hematodinium sp.-Chatton & Poisson 1930-) display alternative cell biological features, meaning derived solutions for conserved tasks. Indeed, apart harbouring huge amount of DNA when compared to mammals, their chromosomes are strikingly peculiar for two reasons: i) they are permanently condensed displaying liquid crystalline characteristics and ii) though they harbour histone encoding genes their protein amount is barely detectable. Consistently, their chromatin is packaged not with histones, but with the help of non-histone basic proteins known as dinoflagellate viral nucleoproteins (DNVPs) [21]. Beside of such kind oddness, it is amazing that such organisms stopped to use histone to package DNA, not because they lost histone genes, but because they acquired new proteins with overriding functions. Noteworthy, when DNVPs are expressed in a host system (i.e., yeast) they displace the endogenous histone [22], suggesting an even higher affinity than that displayed by endogenous histones towards DNA. Overall, these findings lead to some considerations. One above all is: to what extent histones paly role/s other than mediating chromatin condensation? Some insights from their role in guiding the DNA repair machinery to the damaged DNA site have already established, but dinoflagellates suggest that others remain to be unveiled. Though, aquatic unicellular lower eukaryotes look appealing for cell biology inquiries, for quite a long time they have been lagging behind in terms of genetic manipulation. Thanks to the efforts of many scientists, and to the Gordon and Betty Moore foundation EMS program, such a major hindrance has been recently vanquished [23]. With the setting up of growth laboratory conditions and protocols for exogenous DNA delivery a new era has started, letting surmise genome wide screening, functional genomics inquiries alongside their potential future use in biotechnology applications throughout genome-editing.
Conclusions
In the last years marine and aquatic organisms (e.g., sponges, microorganisms) have been regarded as potentially resourceful for many purposes, including drug discovery [24]. In this respect, the rich diversity provided by aquatic protists, alongside that coming from those that continue to be discovered, is vast and in the future hopefully will help us to address unanswered questions in different fields. Besides the detailed understanding of the cell biology of aquatic protists from their very recent genetic manipulation several potential benefits are expected in different fields ranging from aquatic science to evolutionary studies up to medicine and pharmacology. Through the establishment of suitable transformation protocols, by functional genomics and genome-editing it will be possible to shed light on the function of species-specific genes which likely reflect important niche-specific adaptations in dynamic marine ecosystems. Ultimately and noteworthy, the use of aquatic protists as alternative and novel laboratory model organisms fulfill the 3Rs policy, namely, refinement of design aimed to adopt alternative methods that alleviate animal distress, reduction of experiments with mammals whenever possible, and replacement with other approaches. Overall, though we are just experiencing the dawn of this novel era, for aquatic marine protists the outlook for the future is genuinely encouraging.
References
- Davis RH (2004) The age of model organisms. Nat Rev Genet 5(1): 69-76.
- Alfred J, Baldwin IT (2015) New opportunities at the wild frontier. eLife 4: e06956.
- Leonelli S, Ankeny RA (2013) What makes a model organism? Endeavour 37(4): 209-212.
- Sebé-Pedrós A, Degnan BM, Ruiz-Trillo I (2017) The origin of Metazoa: A unicellular perspective. Nat Rev Genet 18(8): 498-512.
- Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on earth. Proc Natl Acad Sci U.S.A. 115(25): 6506-6511.
- Haemmerling J (1934) About form-forming substances in Acetabularia mediterranea, their spatial and temporal distribution and their origin. Wilhelm Roux Arch Develop Mech Org 131(1): 1-81.
- Haemmerling J (1963) Nucleo-cytoplasmic interactions in acetabularia and other cells. Annu Rev Plant Physiol 14: 65-92.
- Winding M, Pedigo BD, Barnes CL, Patsolic HG, Park Y, et al. (2023) The connectome of an insect brain. Science 379(6636): eadd9330.
- Botstein D, Fink GR (2011) Yeast: An experimental organism for 21st century biology. Genetics 189(3): 695-704.
- Kessin RH (2001) Dictyostelium: Evolution, cell biology, and the development of multicellularity. 1st (edn.), Cambridge University Press, USA, pp. 1-385.
- Annesley SJ, Fisher PR (2009) Dictyostelium discoideum-A model for many reasons. Mol Cell Biochem 329(1-2): 73-91.
- Pergolizzi B, Bozzaro S, Bracco E (2017) G-protein dependent signal transduction and ubiquitination in dictyostelium. IJMS 18(10): 2180.
- Bozzaro S, Eichinger L (2011) The professional phagocyte Dictyostelium Discoideum as a model host for bacterial pathogens. CDT 12(7): 942-954.
- Bozzaro S, Buracco S, Peracino B (2013) Iron metabolism and resistance to infection by invasive bacteria in the social amoeba Dictyostelium Discoideum. Front Cell Infect Microbiol 3: 50.
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, et al. (2008) The genome of the Choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451(7180): 783-788.
- Junqueira AC, Yotoko K, Zou H, Friedel RH (2019) Origin and evolution of plexins, semaphorins, and met receptor tyrosine kinases. Sci Rep 9.
- Kirkegaard JB, Bouillant A, Marron AO, Leptos KC, Goldstein RE (2016) Aerotaxis in the closest relatives of animals. eLife 5: e18109.
- Pincus D, Letunic I, Bork P, Lim WA (2008) Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages. Proc Natl Acad Sci USA 105(28): 9680-9684.
- Suga H, Miller WT (2018) Src signaling in a low-complexity unicellular kinome. Sci Rep 8(1): 5362.
- Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, et al. (2008) The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA 105(6): 2070-2075.
- Gornik SG, Ford KL, Mulhern TD, Bacic A, McFadden GI, et al. (2012) Loss of nucleosomal DNA condensation coincides with appearance of a novel nuclear protein in dinoflagellates. Current Biology 22(24): 2303-2312.
- Irwin NAT, Martin BJE, Young BP, Browne MJG, Flaus A, et al. (2018) Viral proteins as a potential driver of histone depletion in dinoflagellates. Nat Commun 9: 1535.
- Faktorová D, Nisbet RER, Fernández Robledo JA, Casacuberta E, Sudek L, et al. (2020) Genetic tool development in marine protists: Emerging model organisms for experimental cell biology. Nat Methods 17(5): 481-494.
- Magnati S, Mortati L, Bracco E (2023) Potential use of marine compounds to modulate the ubiquitin-proteasome system. EIMBO 6(1): 1-4.
© 2023 Enrico Bracco. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






