- Submissions

Full Text
Environmental Analysis & Ecology Studies
Landfill Leachate as a Raw Material: Synthesis, Modification and Characterization of Green Resin
Mahmut Goktas, Melayib Bilgin and Günay Özbay*
Engineering Management, Graduate School of Natural and Applied Sciences, Aksaray University, Turkey
*Corresponding author:Günay Özbay, Engineering Management, Graduate School of Natural and Applied Sciences, Aksaray University, Turkey
Submission: June 13, 2025; Published: July 29, 2025

ISSN 2578-0336 Volume13 Issue 2
Abstract
Managing municipal waste has been an ever-complex and critical common issue worldwide. Municipal waste leads to the generation of landfill Leachate (LL), a highly polluting liquid that is described by its complex composition. This study investigated the probability of utilizing LL as a partial replacement for water in the synthesis of Phenol-Formaldehyde (PF) resol resin. The impact of LL addition on the resin’s physical properties, thermal stability, and bonding quality was investigated. The outcomes demonstrated that the addition of LL influenced several key resin properties, including increased viscosity, decreased pH, increased solids content and free formaldehyde content. Furthermore, the thermal stability of the resins was noticed to decline with increasing LL levels, to be expected due to the presence of organic compounds in the leachate. Both under dry and wet conditions, the bonding quality measurement revealed a decrease in tensile strength for the samples bonded with LL-modified PF resins compared to the control resin. According to these experimental findings, it can be recommended to limit the substitution of pure water with LL in the resin synthesis to a maximum of 20% to mitigate possible negative impacts on resin properties and bonding performance. Finally, LL containing high organic matter can be used in some synthesis processes to add to the economy by minimizing the environmental impact.
Keywords:Landfill leachate; Phenol; Resol resin; Tensile shear strength; TGA analysis
Introduction
Human activities such as transportation, industrial facilities, energy production, and agricultural operations have been proceeding to release considerable amounts of pollutants into the ecology. While environmental pollution remains a concern worldwide, it has been observed a marginal decline in pollution levels in developed countries. This decline can be attributed to the implementation of stricter environmental regulations by local governments [1,2].
Nowadays, there has been notable progress in the application of green waste management technologies. This is fundamentally due to various factors, such as the growing generation of waste resulting from an increasing population and more food consumption. Also, traditional waste management techniques, incineration and landfilling, contribute to pollution, which has raised serious issues in the field of waste management [3-6].
Landfill Leachate (LL) is a multiplex liquid that comprises high contents of biodegradable and non-biodegradable substances, such as organic matter, phenols, ammonia nitrogen, phosphate, heavy metals, and sulphides. If not carefully managed, it can pollute surface water and groundwater [7,8].
Phenol-Formaldehyde (PF) resins are made from polycondensation by phenol and formaldehyde, either under acidic or basic conditions. There are two main types of phenol-Formaldehyde (PF) resins resol and novolac PF resins, depending on the type of catalyst used. PF resol resin is produced at base pH conditions. Especially, the wood products industry has used this resin as an adhesive for its many advantages since the 19th century. The resol resin is recognised for its magnificent mechanical properties, resistance to both heat and chemicals, electrical insulation, and fire retardancy. These properties make it acceptable for a wide range of applications in fields including electronics, machinery, construction and the military [9-13]. However, the use of resol resin has some disadvantages. These involve a short console life, the need for a special catalyst for polymerization, and the release of volatiles during curing, which can result in serious stricture and void formation. Additionally, the resulting products tend to be fragile, and they can pose some healthcare risks owing to the volatilization of phenol and formaldehyde into the air during the curing process [14,15]. Consequently, it has been the subject of numerous research worldwide to produce more environmentally friendly PF resins. These reports are mainly associated with the replacement of phenol with bio-based resources such as lignin and lignin-derived products or pyrolytic-oil [11,16-25]. In this report, landfill leachate (LL) was used as a partial substitute for pure water to prepare a formaldehyde solution. Then, phenol and the formaldehyde solution were synthesized using a base catalyst (NaOH) to obtain a green PF resin. The impact of the LL substitution level on the physical and thermal properties of the PF resol resin was examined. Additionally, the bonding quality of each PF resol resin was measured according to the EN 205 standard (2003).
Materials and Experimental Setup
Leachate liquid and chemicals
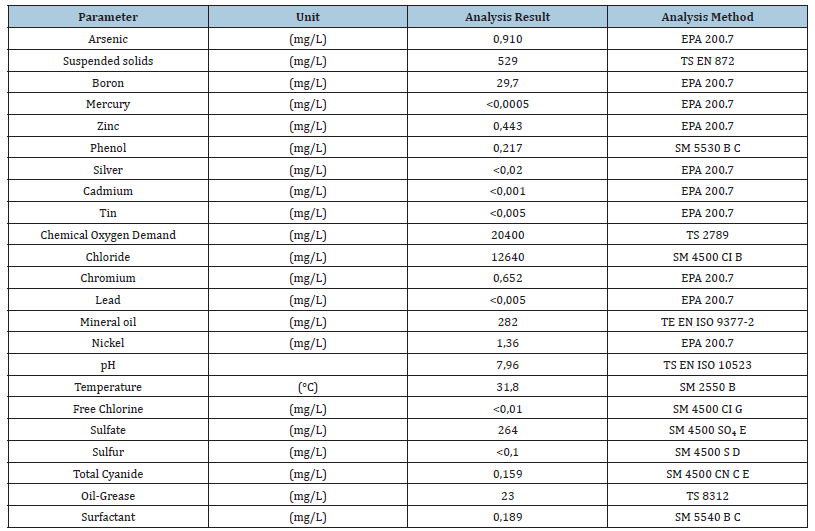
The leachate liquid was collected from a solid waste landfill in Aksaray City, Turkey. Table 1 shows some characteristics of the leachate. Before preparing the formaldehyde solution, the leachate was filtered using filter paper with a mesh size of 100μm. Paraformaldehyde and liquid phenol were obtained from Gentaş Chemical Industry, while the NaOH pellets were purchased from Sigma-Aldrich.
Table 1:Some characteristics of the LL.
Resin synthesis procedure
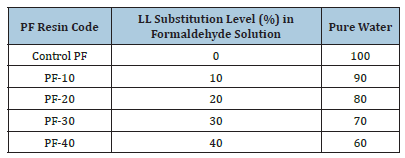
Before synthesizing the PF resol resin, a formaldehyde solution (37% concentration) was prepared with varying addition rates of leachate/pure water (0, 10, 20, 30, and 40wt.%). Different amounts of leachate were mixed with paraformaldehyde and pure water at approximately 500rpm for 10 minutes at room temperature. In the standard synthesis procedure, the phenol/formaldehyde mixture was loaded into the reactor, followed by the addition of 70% of the total NaOH solution (50wt.%) at 60 °C. The mixture was then heated to 90 °C and stirred continuously for 60 minutes. The pH was adjusted to about 11.0 by adding the remaining 30% of the NaOH solution. Finally, after the synthesis was complete, the resol resin was cooled to room temperature (approximately 25 °C). Table 2 outlines the experimental design.
Table 2:The experimental design.
Resol resin characterization
The values of pH and viscosity of the PF resol resins were completed using a pH meter (TES-1380) and a digital viscometer (Brookfield, model: Dv-IPrime) respectively. The Solids Content (SC) of the resol resin was determined following ASTM D 3529- 03. Free Formaldehyde Content (FFC) was measured by the hydroxylamine hydrochloride method (DIN EN ISO 9397-1997). The thermogravimetric analyses were done using the SDT Q600 thermogravimetric analyser. The resol sample (10mg) was heated from 25-700 °C by 10 °C/min in a N2 atmosphere.
Tensile shear strength test procedure
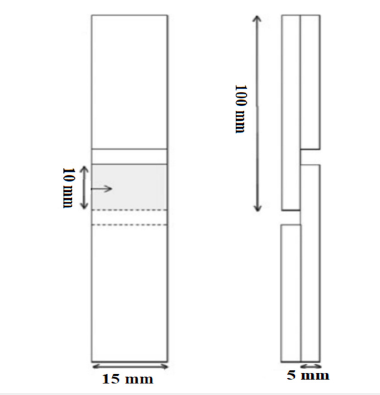
The lamellas were planed to a thickness of 5mm and then bonded together to form a sample. The PF resol resin was applied at a rate of 200g/m² to a single bonding surface of the lamellas. The resol resin was evenly spread on the lamellas using a brush. The press pressure, temperature, and duration were set at 2kg/cm², 120 °C, and 15 minutes, respectively. After conditioning for 8 weeks at 20±2 °C and 65±3% relative humidity, the samples were tested for tensile shear strength using a Zwick/Roel Z50 universal testing machine, according to BS EN 205 and TS EN 12765 standards. The test sample is illustrated in Figure 1.
Figure 1:Tensile shear strength test sample.
Experimental Results Discussion
Physical properties of the PF resol resins
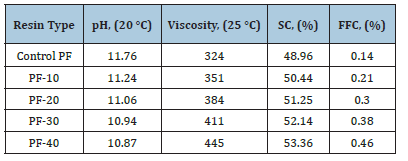
Table 3 presents the values of pH, viscosity, SC, and FFC of the PF resol resins. The data indicate that as the amount of LL rose from 0% to 40% by weight, the pH value of the resol resin reduced from 11.76 to 10.87. The resol resins containing LL (10-40%) exhibited higher viscosity values than the control resol resin. As the amount of LL addition increased, the solid content of the resol resin gradually rose. Additionally, the FFC also increased continually. This is likely due to the higher phenol and organic acid content of LL compared to pure water. In the relevant literature, similar findings have been reported for the use of various bio-based materials in PF resin synthesis [25-29].
Table 3:Some physical properties of the resol resins.
Thermal analysis result
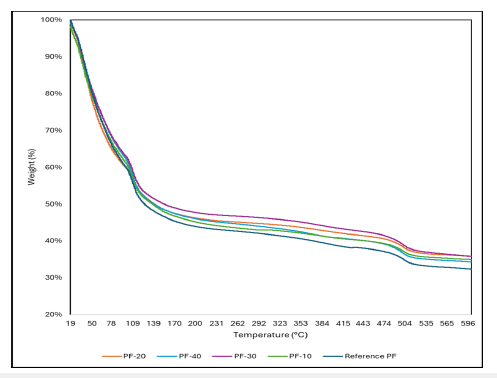
The TGA graphs of the control PF resol resin and the PF resol resins modified with LL are shown in Figure 2. As observed from Figure 2, the thermal degradation of the resol resins modified with different amounts of LL was like that of the control resol resin within the temperature range of 20-700 °C. The mass loss occurs in two main stages. In the first stage, the mass loss occurs over a wide temperature range of 100 to 300 °C. In the first stage, the weight loss could be ascribed to removing water, dehydration, and eliminating volatile compounds. The second mass loss stage occurred in a temperature range of 300 to 600 °C. In stage II, a major weight loss had appeared. It could be attributed to the formaldehyde from the break of ether bonds formed in the curing solution or the dehydrogenation of the methyl groups from the aromatic rings [30-32]. In the past decades, much literature has been reported concerning the thermal degradation of PF resin. The thermal characterization of the PF resins obtained from the TGA analysis agrees with these reported studies [33-37].
Figure 2:Thermal degradation behaviour of control PF resol resin and PF resol resins containing LL.
Bonding performance of the PF resol resin
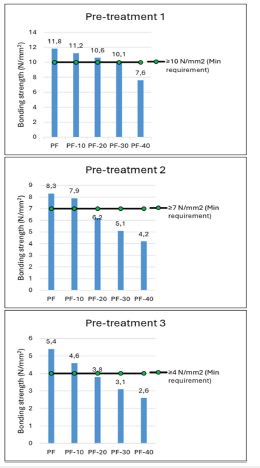
The tensile shear strength test results of the specimens bonded with the reference PF and PF resins containing LL are presented in Figure 3. Under dry conditions (pre-treatment 1), as the content of the LL was raised to 10%wt., the strength of the samples slightly decreased from 11.8 to 11.2 N/mm2. Based on the EN 12765 standard, the minimum tensile strength must be 10N/ mm2 for pre-treatment 1. The wood samples bonded with modified PF resins up to 30%wt. LL met the requirements of the standard. Figure 3 displays the shear strength values of the test samples for pre-treatment 2. The shear strength values of all the test samples ranged from 8.3 to 4.2N/mm2. In the evaluation of pre-treatment 3, as the amount of LL in the PF resin was increased up to 20%wt., the strength of the samples decreased from 5.4 to 3.8N/mm2. Also, the samples bonded with PF-20, PF-30, and PF-40 could not exceed the minimum requirements for class C3 (EN 12765). The results of tensile strength tests showed that the decrease in the bonding performance of the PF resin decreased above a certain concentration (20%wt.) of LL under both dry and wet conditions. Two factors could explain this result: the amounts of organics derived from the LL suspension and the increase in the viscosity of the PF resin containing LL [38].
Figure 3:The tensile shear strength values of PF resins (N/mm2).
Conclusion
This study used LL, a raw material rich in organic content, to
partially replace pure water in synthesising phenol-Formaldehyde
(PF) resin. The influence of the LL substitution rate on the thermal
properties of the PF resin was assessed by thermogravimetric
analysis. Additionally, the bonding quality of each PF resol resin was
evaluated through tensile strength tests. The following conclusions
can be drawn:
A. The presence of LL affected several physical properties of the
PF resol resins, including viscosity, pH value, SC, and FFC. With
the addition of LL, the pH values of the resol resins revealed a
modest decreasing trend. Conversely, the viscosity, SC, and FFC
of the resol resins exhibited an increasing trend.
B. The thermal behaviour of the control resol resin was more
stable than the resol resins containing various amounts of
LL. At temperatures exceeding 300 °C, it can be claimed that
some organics within the LL negatively impacted the thermal
stability of the PF resol resin.
C. Increased LL content in the PF resin generally decreased the
tensile strength of the wood samples under dry conditions. For
wet conditions, the tensile strength of samples bonded with
PF resins containing LL exhibited lower bonding performance
compared to those bonded with the reference PF resin after
pre-treatment 2 and 3 tests. It is recommended to partially
substitute LL (up to 20%) for water in the production of PF
resins to minimize negative impacts on resin properties and
bonding performance.
Acknowledgement
This study was made as a master’s thesis by Mahmut GOKTAS in the Institute of Natural and Applied Sciences, Aksaray University, Aksaray, 2024.
References
- Kamal AKI, Islam MR, Hassan M, Ahmed F, Rahman T, et al. (2016) Bioaccumulation of trace metals in selected plants within amin bazar landfill site, Dhaka, Bangladesh. Environ Process 3: 179-194.
- Mishra S, Tiwary D, Ohri A, Agnihotri AK (2019) Impact of municipal solid waste landfill leachate on groundwater quality in Varanasi, India. Groundw Sustain Dev 9: 100230.
- Guerreiro C, Foltescu V, de Leeuw F (2014) Air quality status and trends in Europe. Atmospheric Environment 98: 376-384.
- Fayiga AO, Ipinmoroti MO, Chirenje T (2018) Environmental pollution in Africa. Environment, development and sustainability 20: 41-73.
- Mariyam S, Alherbawi M, Pradhan S, Al-Ansari T, McKay G (2023) Biochar yield prediction using response surface methodology: effect of fixed carbon and pyrolysis operating conditions. Biomass Conversion and Biorefinery 14: 28879-28892.
- Budihardjo MA, Priyambada IB, Chegenizadeh A, Al Qadar S, Puspita AS (2023) Environmental impact technology for life cycle assessment in municipal solid waste management. Global Journal of Environmental Science and Management 9(Special Issue): 145-172.
- Kamaruddin MA, Yusoff MS, Aziz HA, Hung YT (2015) Sustainable treatment of landfill leachate. Applied Water Science 5: 113-126.
- Salem Z, Hamouri K, Djemaa R, Allia K (2008) Evaluation of landfill leachate pollution and treatment. Desalination 220(1-3): 108-114.
- Wolff R, Ehrmann K, Knaack P, Seidler K, Gorsche C, et al. (2022) Photo-chemically induced polycondensation of a pure phenolic resin for additive manufacturing. Polymer Chemistry 13(6): 768-777.
- Xu Y, Guo L, Zhang H, Zhai H, Ren H (2019) Research status, industrial application demand and prospects of phenolic resin. RSC Advances 9(50): 28924-28935.
- Kokten ES, Özbay G, Ayrilmis N (2018) Synthesis of biobased phenolic resins using catalytic pyrolysis oil and its effect on oriented strand board performance. The Journal of Adhesion 96(5): 475-489.
- Sandomierski M, Buchwald T, Strzemiecka B, Voelkel A (2020) Carbon black modified with 4-hydroxymethylbenzenediazonium salt as filler for phenolformaldehyde resins and abrasive tools. J Appl Polym Sci 137(3): 48160.
- Marliana MM, Hassan A, Yuziah MN, Khalil HA, Inuwa IM, et al. (2016) Flame retardancy, thermal and mechanical properties of kenaf fiber reinforced unsaturated polyester/phenolic composite. Fibers and Polymers 17: 902-909.
- Takeichi T, Kawauchi T, Agag T (2008) High performance polybenzoxazines as a novel type of phenolic resin. Polymer Journal 40(12): 1121-1131.
- Özbay G, Cekic C, Ahmad MS, Kokten ES (2020) Synthesis of bio-oilphenol-formaldehyde resins under alkali conditions: Physical, chemical and thermal properties of resins and bonding performance. Wood Industry 71(1): 19-27.
- Park Y, Doherty WOS, Halley PJ (2008) Developing lignin-based resin coatings and composites. Industrial Crops and Products 27(2): 163-167.
- Jin Y, Cheng X, Zheng Z (2010) Preparation and characterization of phenol–formaldehyde adhesives modified with enzymatic hydrolysis lignin. Bioresource Technology 101(6): 2046-2048.
- Pang B, Yang S, Fang W, Yuan TQ, Argyropoulos DS, et al. (2017) Structure-property relationships for technical lignins for the production of lignin-phenol-formaldehyde resins. Industrial Crops and Products 108: 316-326.
- Kouisni L, Fang Y, Paleologou M, Ahvazi B, Hawari J, et al. (2011) Kraft lignin recovery and its use in the preparation of lignin-based phenol formaldehyde resins for plywood. Cellulose Chemistry and Technology 45(7): 515.
- Venkatesagowda B, Dekker RF (2020) Enzymatic demethylation of Kraft lignin for lignin-based phenol-formaldehyde resin applications. Biomass Conversion and Biorefinery 10(2): 203-225.
- Peng Z, Jiang X, Si C, Cárdenas‐Oscanoa AJ, Huang C (2023) Advances of modified lignin as substitute to develop lignin‐based phenol‐formaldehyde resin adhesives. ChemSusChem 16(15): e202300174.
- Choi GG, Oh SJ, Lee SJ, Kim JS (2015) Production of bio-based phenolic resin and activated carbon from bio-oil and biochar derived from fast pyrolysis of palm kernel shells. Bioresource Technology 178: 99-107.
- Yu Y, Xu P, Chen C, Chang J, Li L (2018) Formaldehyde emission behavior of plywood with phenol-formaldehyde resin modified by bio-oil under radiant floor heating condition. Building and Environment 144: 565-572.
- Celikbag Y, Nuruddin M, Biswas M, Asafu-Adjaye O, Via BK (2020) Bio-oil-based phenol-formaldehyde resin: Comparison of weight-and molar-based substitution of phenol with bio-oil. Journal of Adhesion Science and Technology 34(24): 2743-2754.
- Yu Y, Qiu X, Li C, Bao D, Chang J (2023) Performance and characterization of phenol-formaldehyde resin with crude bio-oil by model compound method. Plos One 18(1): e0271478.
- Lee WJ, Chang KC, Tseng IM (2012) Properties of phenol‐formaldehyde resins prepared from phenol‐liquefied lignin. Journal of Applied Polymer Science 124(6): 4782-4788.
- Qiao W, Li S, Xu F (2016) Preparation and characterization of a phenol-formaldehyde resin adhesive obtained from bio-ethanol production residue. Polymers and Polymer Composites 24(2): 99-105.
- Li Q, Liu X, Su H, Mao A, Wan H (2020) Improving performance of phenol-formaldehyde resins modified/blended with phenol-rich pyrolysis bio-oil. Forest Products Journal 70(4): 387-395.
- Lv S, Huang Y, Gao Z, Hou X, Yang J, et al. (2024) Waste sawdust as a raw material: Synthesis, modification and characterization of novel bio-based phenol-formaldehyde wood adhesives. Industrial Crops and Products 219: 119067.
- Xu LY, Shi ZG, Feng YQ (2008) Preparation of a carbon monolith with bimodal perfusion pores. Microporous Mesoporous Mater 115(3): 618-623.
- Ozaki J, Ohizumi W, Oya AA (2000) TG-MS study of ploy (vinyl butyral)/phenol-formaldehyde resin blend fiber. Carbon 38(10): 1515-1519.
- Yuan Z, Hu Z, Wu R, Liu Q (2012) The pyrolysis mechanism of phenol formaldehyde resin. Polym Degrad Stab 97(8): 1527-1533.
- Wong HW, Peck J, Bonomi RE, Assif J, Panerai F, et al. (2015) Quantitative determination of species production from phenol-formaldehyde resin pyrolysis. Polymer Degradation and Stability 112: 122-131.
- Park BD, Kadla JF (2012) Thermal degradation kinetics of resole phenol-formaldehyde resin/multi-walled carbon nanotube/cellulose nanocomposite. Thermochimica Acta 540: 107-115.
- O'Connor D, Blum, FD (1987) Thermal stability of substituted phenol‐formaldehyde resins. Journal of Applied Polymer Science 33(6): 1933-1941.
- Tejado A, Pena C, Labidi J, Echeverria JM, Mondragon I (2007) Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresource Technology 98(8): 1655-1663.
- Alonso MV, Oliet M, Dominguez JC, Rojo E, Rodriguez F (2011) Thermal degradation of lignin-phenol-formaldehyde and phenol-formaldehyde resol resins: structural changes, thermal stability, and kinetics. Journal of Thermal Analysis and Calorimetry 105(1): 349-356.
- Ghosh NN, Kiskan B, Yagci Y (2007) Polybenzoxazines-new high performance thermosetting resins: Synthesis and properties. Progress in Polymer Science 32(11): 1344-1391.
© 2025 © Günay Özbay. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






