- Submissions

Full Text
Environmental Analysis & Ecology Studies
Transcriptomic Analysis of AREB1 and AREB2 Genes Playing Important Roles in Drought Stress Tolerance in Tomato under in vitro Drought Stress
Çiçek S, Ağar H, Galatalı S and Kaya E*
Molecular Biology and Genetic Department, Faculty of Science, Muğla Sıtkı Koçman University, Turkey
*Corresponding author:Kaya E, Molecular Biology and Genetic Department, Faculty of Science, Muğla Sıtkı Koçman University, Turkey
Submission: March 17, 2023; Published: April 18, 2023

ISSN 2578-0336 Volume10 Issue5
Abstract
Solanum lycopersicum (tomato), which is one of the important agricultural products in the world and in also Turkey, is a valuable plant in food industry, with its high content of vitamins, minerals and flavonoids. Usable water resources are decreasing over time due to reasons such as unconscious water consumption, wrong agricultural treatments and uncontrolled use of chemicals (artificial fertilizers, herbicides/pesticides) in the world. The drought, which has recently emerged as one of the biggest problems in the world, poses a great threat to plants. Plants respond to such stresses in different ways. Together with these stress responses, they show maximum adaptability and resilience. After the stress factor is perceived by plants, many stress-specific signal transduction pathways and gene regulation are activated/inactivated. In the current study, it was aimed to investigate the mRNA level of SlAREB1 and SlAREB2 genes, which are thought to play a role in drought stress tolerance in tomato plants. Quantitative PCR was performed in the presence of two genes thought to be effective in drought tolerance and positive control in S. lycopersicum plant grown in media containing different PEG concentrations in in vitro. Thus, it has been confirmed that these genes expressed against drought stress are effective on tomato plants. This study will form the basis for future studies. It will be important in identifying other yet undiscovered genes that are effective in the metabolic pathways of drought stress. CT values obtained after β-actin normalization with 2-ΔΔCT method were proportioned to the control group and a graph was created. As a result of T-TEST with the results obtained, it was observed that there was a significant increase in 5% PEG and 10% PEG concentrations for the SlAREB1 gene compared to the control group. There was no significant increase or decrease in 2.5% and 20% PEG concentrations compared to the control group. For the SlAREB2 gene, there was a significant increase in 2.5% PEG, 5% PEG and 10% PEG concentrations compared to the control group. There was no significant increase or decrease in 20% PEG concentration.
Keywords:Abiotic stress; Polyethylene Glycol (PEG); AREB genes; Real-Time PCR
Introduction
Tomato (S. lycopersicum) belonging to a large family, Solanaceae, is one of the most produced, consumed and exported agricultural products in the world and Turkey. After its emergence, it has become important as a food and has been bred for purposes such as overproduction, increasing fruit quality and being able to be grown in different environments [1]. It has been reported that it has antioxidant and anticancer properties and is considered as a protective food, thanks to its rich content such as vitamins A, E, C, lycopene, beta-carotene (provitamin A), folate, and flavonoids [2,3]. The consumption of tomato, which ranks 7th in worldwide production, is 20kg per person per year [2,4]. Turkey is among the important countries in tomato production in the world due to its favorable climatic conditions. Turkey ranks third in the world with a total tomato production of 12.7 million tons in recent years. According to 2018 data, Turkey meets 7.2% of the total tomato production in the world [5].
With unconscious water consumption, uncontrolled use of pesticides, wrong agricultural practices, climate changes and global warming, usable water resources, which are in short quantities due to population-related demand, are endangered. These usable water resources, which are small compared to the total amount of water; It is decreasing over time due to overpopulation, water pollution and increase in greenhouse gases. Due to all these factors, drought, which is one of the biggest global problems of today, occurs [6,7]. Abiotic stress factors such as sudden increases and decreases in temperatures, drought, excessive salinity, excess or insufficient amount of soil nutrients and UV rays as a result of climatic changes adversely affect the growth, development and reproduction of the plant [8,9]. These adverse environmental conditions create stress on plants and are called abiotic stress factors. It limits the vital activities of the plant such as germination, growth, development and reproduction [10].
Drought has recently emerged as one of the biggest problems in the world and our country and poses a threat to plants [11]. However, it severely limits agricultural production and crop yield. When plants are exposed to less water than they need, they create stress responses that maintain vital activities by undergoing or regulating morphological, physiological and biochemical changes [12]. Many mechanisms such as closure of stomata, reduction of water loss, greater and deeper rooting, preservation of membrane integrity, antioxidant defense system, change in the functioning of plant growth regulators, aquaporin and stress-related protein activity are effective in resistance to drought [13]. Plants show maximum adaptation and endurance with these stress responses as well as developmental flexibility mechanisms in adapting to environmental conditions [14].
After stress factor detection, plant cells activate/inactivate many signal transduction systems and gene regulation specific to that stress. These molecular mechanisms, which form the basis of all changes developed against stress, are potential targets for growing plants under stress conditions and crop yield [8]. Molecular studies to increase the stress resistance of economically valuable plants such as tomatoes are important in terms of elucidating the stress response mechanisms and developing possible resistance strategies [13,15]. Signaling pathways activated in stress responses and transcription factors associated with these pathways regulate gene expression. Abscisic acid (ABA), an endogenous phytohormone, has been reported to increase significantly in most plants exposed to drought stress [16]. Many transcription factors regulate gene expression in association with ABA. The ABA-dependent stress response is mediated by abscisic acid-sensitive element (ABRE) binding proteins (AREB) [17]. Two AREB (SIAREB1 and SIAREB2) transcription factors expressed in roots, stems, leaves and fruits have been found to be involved in the regulation of stress responserelated genes [18].
In the present study, it was aimed to investigate the transcriptional level of SlAREB1 and SlAREB2 genes, which are thought to be involved in the tolerance mechanisms against drought stress as a result of in vitro transfer of tomato to the environment free from microorganisms and then in vitro drought stress application created to use PEG in these conditions. In addition, it was also aimed to determine the concentration range that the plant can tolerate drought stress. In this context, all these findings will play a key role in the determination of other, yet unidentified genes involved in drought tolerance in tomatoes, which are planned to be made in the future. In addition, this study will contribute to the literature as a useful reference for other researchers working in similar fields.
Materials and Method
Plant material
S. lycopersicum seeds were obtained from Muğla Metropolitan Municipality Local Seed Center. The seeds were surface sterilized according to protocols of Ozudogru EA et al. [19] & Kaya E et al. [20]. For this purpose, they were washed under tap water for 15 minutes. After the washing process, the seeds taken into the laminar flow cabinet were treated with 70% EtOH for 5 minutes and 10% commercial bleach (Domestos®) for 10 minutes, respectively. After each chemical treatment, the seeds were rinsed with distilled water. After sterilization, the seeds were dried on sterile filter paper.
The surface sterilized seeds were taken into MS [21] medium containing 1mgL-1 6-Benzylaminopurine (BA). They were incubated for 4-6 weeks at 27±2 °C temperature, 16/8 hour photoperiod and 50μmol-1 m-2 s -1 white daylight fluorescently illuminated at standard culture conditions [22]. For in vitro mass propagation of S. lycopersicum micro-shoots after germination and development stage, the micro-shoots were obtained with at least a binodal segment from each plant material under sterile conditions then, they were transferred to MS medium containing 1mgL-1 BA [23]. It was incubated for 4-6 weeks at standard culture conditions described above.
Drought treatment
The shoot tips of S. lycopersicum, which were adequately micro propagated, were transferred to MS [21] media containing 0%, 2.5%, 5%, 10% or 20% Polyethylene Glycol (PEG). Plants were exposed to drought stress by incubating them for 4 weeks under ambient conditions of 27±2 °C, a photoperiod of 16/8 hours and illuminated with 50μmol-1 m-2 s -1 white daylight fluorescence [24,25].
Molecular analyzes
Total RNA was isolated from the leaves and stems of plant explants exposed to drought stress with PEG application with Thermo Scientific GeneJET Plant RNA Purification Kit. Electrophoretic and spectrophotometric analyzes were performed to measure the amount and quality of the RNA obtained. The obtained RNAs were reverse transcribed with the OneScript® Plus Reverse Transcriptase OneScript® cDNA Synthesis kit and the oligoDT primers included in this kit. Real-Time PCR was performed in accordance with the AmpliqonRealQ Plus 2×Master MixGreen kit protocol with the primers provided by reference to previous studies [16] on S. lycopersicum plant. Expression levels of target genes were determined under the control of reference genes. The significance of the expression levels of the genes of interest in the results obtained after Real Time PCR compared to the control group was statistically analyzed by T-Test method. Among the data obtained as a result of the T-Test, those with p≤0.05 were considered statistically significant [15].
Results and Discussion
In vitro propagation of Solanum lycopersicum L
The seeds of the S. lycopersicum used in the current study were transferred to the in vitro conditions after surface sterilization and approximately 100% of seeds germinated successfully (Figure 1). S. lycopersicum seeds, which germinated successfully in in vitro, were subcultured on MS for drought treatment with adding PEG for four-week periods to obtain sufficient plant material. Germination is affected by internal and environmental factors. Environmental factors play an important role in stimulating seeds for germination or entering a dormant period. This effect is mostly due to changes in the levels of plant hormones (plant growth regulators) [20,26,27]. BA used as a growth regulator in the present study had positive effects on germination.
Figure 1:General view of S. lycopersicum seeds germinated in in vitro conditions during 2-4 weeks incubation.

Effects of drought stress on plant growth
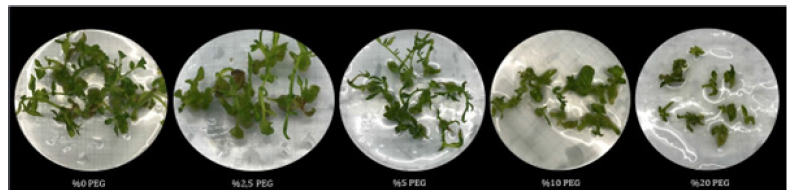
The micro-shoots of the in vitro grown S. lycopersicum were morphologically examined after incubation for 2-4 weeks in MS semi-solid medium containing 0%, 2.5%, 5%, 10% or 20% PEG. When the groups formed with different concentrations of PEG for drought stress were examined morphologically, it was observed that plant growth was negatively affected at increasing concentrations of PEG (Figure 2) compared to the control group. It was observed that the growth in leaf areas and stem lengths decreased depending on the increase in the treated PEG concentration. It was also observed that the growth rate was very low and the plant loss was high, especially in the medium containing 20% PEG. The growth and development of plants are under the influence of environmental factors such as salinity and drought. Adverse environmental conditions reduce vegetative growth and yield in plants, decrease fruit size and quality, and also cause economic losses [28]. The negative effects of in vitro drought stress applied in our study were observed quite clearly. In a study conducted by Şimşek O et al. [29], the growth performances of two Citrus cultivar (C-35 and Troyer) rootstocks at different PEG doses of both genotypes were found to be statistically significant.
Figure 2:The image of S. lycopersicum cultured in MS medium containing different PEG concentrations (0%, 2.5%, 5%, 10% or 20%) for 2-4 weeks.
Effects of drought stress on transcriptomics
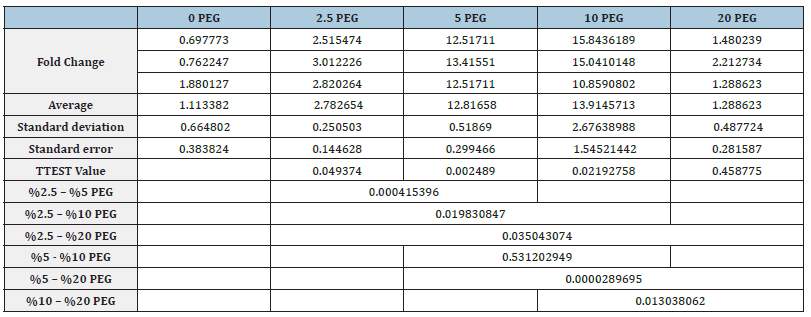
Quantitative PCR results performed in the presence of primers belonging to two genes thought to be effective in drought stress tolerance in S. lycopersicum plant and β-actin gene as positive control and the number of cycles (CT values) corresponding to the point where the relevant genes cross the threshold value in drought stressed plants are given in Table 1. CT values obtained as a result of Real-Time PCR performed to determine the expression level of two different genes in the experimental groups compared to the control group under stress conditions were evaluated in the presence of negative controls. Each experimental group was compared to the control group and interpreted graphically. In the method used, CT values were normalized with the β-actin gene, which was evaluated under the same conditions and used as a positive control. The significance of the experiments was evaluated with the T-Test by performing two experiments with three replicates for each gene. Among the data obtained as a result of the T-Test, those with a value of p≤0.05 were considered significant.
Table 1:CT values obtained after Real-Time PCR with drought stressed plants.
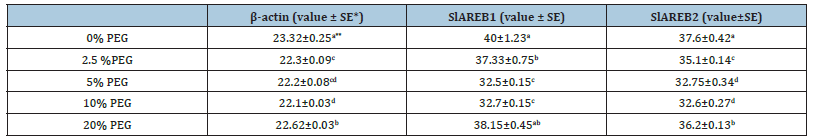
*Values followed by the same letter within each variable and cultivar area are not significantly different (p<0.05), according to the LSD test. **SE, Standard Error
LEA (Late-embryogenesis abundant) proteins are watersoluble proteins synthesized in high concentration in desiccationtolerant plants. It has been reported that dehydrin and ferritin proteins are upregulated in an increased direction in soybean plants under drought stress [30]. Dehydrins are LEA proteins and can effectively increase plant growth under stress by reducing the harmful effects of reactive oxygen species [31]. It has been reported that many proteins detected in Medicago truncatula plants are LEA proteins and these proteins are associated with drought tolerance [32].
Investigation of SlAREB1/2 gene expression level in the occurrence of drought stress in S. lycopersicum
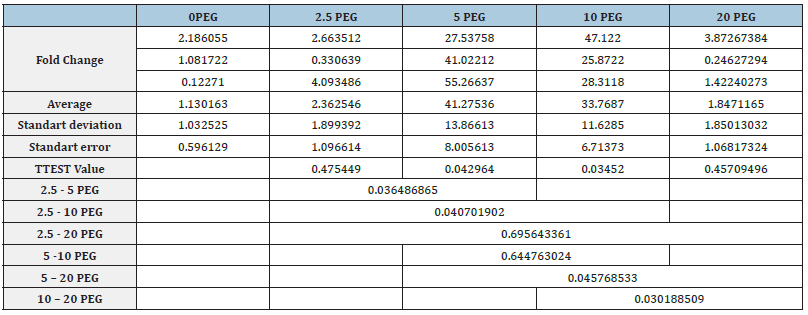
In order to determine the expression level of SlAREB1 gene in five experimental groups together with the control group, Real- Time PCR was performed in triplicate and two experiments. The mean, standard deviation and standard errors of the obtained CT values were calculated (Table 2). CT values obtained after β-actin normalization with 2-ΔΔCT method were proportioned to the control group and a graph was created. As a result of T-TEST performed to see the significance of the results obtained, it was observed that the expression level of the SlAREB1 gene increased significantly in plants exposed to drought stress at 5% PEG and 10% PEG concentrations compared to the control group. As a result of T-TEST, there was no significant increase or decrease in plants exposed to drought stress at 2.5% PEG and 20% PEG concentrations compared to the control group (Figure 3).
Figure 3:Relative mRNA expression graph of SlAREB1 gene.
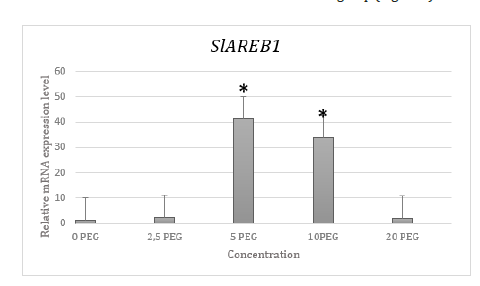
*, p ≤ 0,05; **, p ≤ 0,01; *** p ≤ 0,001
Table 2:Means, standard deviations, standard error values and T-TEST results of CT values of SlAREB1 gene after normalization with β-actin
Abscisic Acid (ABA) signal has a very important role in plant stress response. The studies of drought-induced genes have shown that these genes are stimulated by ABA. The Transcription Factor (TF) families, bZIP and MYB, are involved in ABA signaling and its gene activation. Most ABA-stimulated genes share the ACGTGGC motif in the cis-activating region (C/T) of the promoter of the ABAResponsive Element (ABRE) [33,34].
Under water-limited cellular dehydration conditions, an elevation of endogenous ABA level is induced. In this case, downstream targets of genes encoding signaling factors and transcription factors are stimulated. Acquiring ABA-related plant stress tolerance is effective in drought stress as well as dehydration stress. ABF3 and ABF4 expression; In Arabidopsis thaliana, rab18 increased drought tolerance by altering the expression of ABA/ stress-stimulated genes such as ABI1 and ABI2 [34,35]. Similarly, in the current study, the stress caused by PEG at increased rates (5%) caused an increase in the transcription of the SlAREB1 gene to some extent. In order to determine the expression level of SlAREB2 gene in five experimental groups together with the control group, Real-Time PCR was performed in triplicate and two experiments. The mean, standard deviation and standard errors of the obtained CT values were calculated (Table 3).
Table 3:Means, standard deviations, standard error values and T-TEST results of CT values of SlAREB2 gene after normalization with β-actin.
PEG: Polyethylene glycol.
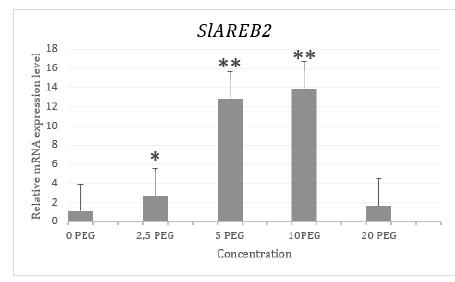
Figure 4:Relative mRNA expression plot of the SlAREB2 gene.
*, p ≤ 0,05; **, p ≤ 0,01; *** p ≤ 0,001
CT values obtained after β-actin normalization with 2-ΔΔCT method were proportioned to the control group and a graph was created. As a result of T-TEST performed to see the significance of the results obtained, it was observed that the expression level of the SlAREB2 gene increased significantly in plants exposed to drought stress at 2.5% PEG, 5% PEG and 10% PEG concentrations compared to the control group. As a result of T-TEST, no significant increase or decrease was observed in plants exposed to drought stress at 20% PEG concentration compared to the control group (Figure 4).
It has been determined that in vitro drought stress conditions, their micropropagation performances continue to live and multiply at increasing PEG doses, but their performance decreases. When plants are exposed to stress, stress signals are usually sensed through special receptors and then these signals are sent to the signal transduction mechanism to regulate gene expression [36]. In the present study, an increase in the transcription of the relevant stress genes was observed when the PEG concentration was increased to some extent. If this stress factor is applied more than the plant can tolerate, the plant may have suppressed the return of these genes in order to survive.
Conclusion
In this study, the expressions of mRNA levels of SlAREB1 and SlAREB2 genes, which are thought to play a role in drought stress tolerance in tomato plants, were investigated. Quantitative PCR was performed in the presence of two genes thought to be effective in drought tolerance and positive control in tomatoes grown on media containing different PEG concentrations in vitro. According to the results of the study, significant increases were observed in the concentrations of 2.5% PEG, 5% PEG and 10% PEG for the SlAREB2 gene compared to the control group. No significant increase or decrease in 20% PEG concentration was observed. During drought stress, various defense mechanisms are regulated at physiological, biochemical and molecular levels. Drought stress affects plant growth by affecting various physiological and biochemical processes such as photosynthesis, respiration, translocation, ion uptake, water potential, stomatal closure, sugar and nutrient metabolism, antioxidant system, and also phytohormones [37,38]. Many proteomic studies have shown that some protein classes are promoted in response to drought stress and that these proteins may play an important role in defense and adaptation processes [39- 41]. In the current study, it was aimed to examine the expressions of two different genes at the transcription level in response to drought stress. The use of transcriptomic analyses and genomic information will facilitate the discovery of new candidate proteins and the understanding of tolerance-related dynamics. In addition, with the development of proteome analysis technologies in the future, a better understanding of post-translational protein modifications, protein-protein interactions and molecular networks, which have important roles in the response to drought stress, will contribute to the development of new plant genotypes that can easily grow in arid regions.
Acknowledgement
The plant material of this work was provided by the Muğla Metropolitan Municipality Agricultural Services Department.
References
- Kimura S, Sinha N (2008) Tomato (Solanum lycopersicum): A model fruit-bearing crop. CSH Protoc 2008(11): pdb-emo105.
- Gerszberg A, Hnatuszko-Konka K, Kowalczyk T, Kononowicz AK (2015) Tomato (Solanum lycopersicum L.) in the service of biotechnology. Plant Cell Tissue Organ Cult (PCTOC) 120: 881-902.
- Wai AH, Naing AH, Lee DJ, Kim CK, Chung MY (2020) Molecular genetic approaches for enhancing stress tolerance and fruit quality of tomato. Plant Biotechnol Rep 14: 515-537.
- Li Y, Wang H, Zhang Y, Martin C (2018) Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites? Plant Cell Rep 37(10): 1443-1450.
- Arslan S, Arısoy H, Karakayacı Z (2022) The situation of regional concentration of tomato foreign trade in Turkey. Turkish Journal of Agriculture-Food Science and Technology (TURJAF) 10(2): 280-289.
- O’Connell E (2017) Towards adaptation of water resource systems to climatic and socio-economic change. Water Resour Manag 31: 2965-2984.
- Seleiman MF, Al-Suhaibani N, Ali N, Akmal M, Alotaibi M, et al. (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 10(2): 259.
- Zhang H, Zhu J, Gong Z, Zhu JK (2022) Abiotic stress responses in plants. Nat Rev Genet 23(2): 104-119.
- Galatali S (2022) Cold stress: Molecular effects on medicinal plants. Turkish Journal of Scientific Reviews 15(2): 63-78.
- Imran QM, Falak N, Hussain A, Mun BG, Yun BW (2021) Abiotic stress in plants; stress perception to molecular response and role of biotechnological tools in stress resistance. Agronomy 11(8): 1579.
- Iqbal MS, Singh AK, Ansari MI (2020) Effect of drought stress on crop production. In: Rakshit A, Singh H, Singh A, Singh U, Fraceto L (Eds.), New Frontiers in Stress Management for Durable Agriculture, Springer, Singapore, pp. 35-47.
- Yang X, Lu M, Wang Y, Wang Y, Liu Z, et al. (2021) Response mechanism of plants to drought stress. Horticulturae 7(3): 50.
- Kapoor D, Bhardwaj S, Landi M, Sharma A, Ramakrishnan M, et al. (2020) The impact of drought in plant metabolism: How to exploit tolerance mechanisms to increase crop production. Appl Sci 10(16): 5692.
- Waadt R, Seller CA, Hsu PK, Takahashi Y, Munemasa S, et al. (2022) Plant hormone regulation of abiotic stress responses. Nat Rev Mol Cell Biol 23(10): 680-694.
- Galatali S, Kaya E (2022) Investigation of the cold stress effect on mac_4 (HSP80-Like) gene at transcriptional level for Mentha × piperita L. Mod Concep Dev Agrono 10(5): 1057-1059.
- Xu Z, Wang F, Ma Y, Dang H, Hu X (2022) Transcription factor SlAREB1 is involved in the antioxidant regulation under saline-alkaline stress in tomato. Antioxidants 11(9): 1673.
- Orellana S, Yanez M, Espinoza A, Verdugo I, Gonzalez E, et al. (2010) The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress‐related genes in tomato. Plant Cell Environ 33(12): 2191-2208.
- Mou W, Li D, Luo Z, Li L, Mao L, et al. (2018) SlAREB1 transcriptional activation of NOR is involved in abscisic acid-modulated ethylene biosynthesis during tomato fruit ripening. Plant Sci 276: 239-249.
- Ozudogru EA, Kaya E, Kirdok E, Issever-Ozturk S (2011) In vitro propagation from young and mature explants of thyme (Thymus vulgaris and longicaulis) resulting in genetically stable shoots. In Vitro Cell Dev Biol-Plant 47(2): 309-320.
- Kaya E, Souza FVD, Yılmaz Gökdoğan E, Ceylan M, Jenderek M (2017) Cryopreservation of citrus seed via dehydration followedby immersion in liquid nitrogen. Turk J Biol 41(1): 242-248.
- Murashige T, Skoog FA (1962) Revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15(3): 473-497.
- Kaya E, Balci MA, Akguller O, Galatali S, Yeniocak S, et al. (2021) Development of an optimum proliferation medium via the graph kernel statistical analysis method for genetically stable in vitro propagation of endemic Thymus cilicicus (Turkey). Acta Bot Croat 80(2): 199-207.
- Kivrak K, Galatali S, Yeniocak S, Ozkaya DE, Mercan T, et al. (2021) Investigation of modified WPM medium for the best meristem proliferation of Corylus avellana L. Adv Hortic Sci 35(3): 285-292.
- Joshi R, Shukla A, Sairam RK (2011) In vitro screening of rice genotypes for drought tolerance using polyethylene glycol. Acta Physiol Plant 33: 2209-2217.
- Mercan T, Galatalı S, Özkaya DE, Çelik O, Kaya E (2022) Effects of different boron salt treatments on micropropagation and genetic stability in in vitro cultures of Liquidambar orientalis Journal of Boron 7(4): 521-527.
- Zeng S, Zhang Y, Teixeira da Silva JA, Wu K, Zhang J, et al. (2014) Seed biology and in vitro seed germination of Cypripedium. Crit Rev Biotechnol 34(4): 358-371.
- Kaya E, Souza FVD, dos Santos-Serejo JA, Galatali S (2020) Influence of dehydration on cryopreservation of Musa spp. germplasm. Acta Bot Croat 79(2): 99-104.
- Agar H, Galatali S, Ozkaya DE, Kaya E (2022) A primary study: Investigation of the in vitro salt stress effects on development in Thymus Cilicicus Boiss & Bal. Glob J Bot Sci 10: 23-27.
- Şimşek Ö, Dönmez D, Aka Kaçar Y (2018) Investigation into performance of some citrus rootstocks in in vitro drought stress conditions. YYU J Agr Sci 28(3): 305-310.
- Alam I, Sharmin SA, Kim K, Yang JK, Choi MS, et al. (2010) Proteome analysis of soybean roots subjected to short-term drought stress. Plant Soil 333: 491-505.
- Hossain Z, Khatoon A, Komatsu S (2013) Soybean proteomics for unraveling abiotic stress response mechanism. J Proteome Res 12(11): 4670-4684.
- Boudet J, Buitink J, Hoekstra FA, Rogniaux H, Larré C, et al. (2006) Comparative analysis of the heat stable proteome of radicles of Medicago truncatula seeds during germination identifies late embryogenesis abundant proteins associated with desiccation tolerance. Plant Physiol 140(4): 1418-1436.
- Agarwal PK, Agarwal P, Reddy MK, Sopory SK (2006) Role of DREB transcription factors in abiotic and biotic stress tolerance in plants. Plant Cell Rep 25(12): 1263-1274.
- Bhatnagar-Mathur P, Vadez V, Sharma K (2008) Transgenic approaches for abiotic stress tolerance in plants: Retrospect and prospects. Plant Cell Rep 27(3): 411-424.
- Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 218(1): 1-14.
- Yan SP, Zhang QY, Tang ZC, Su WA, Sun WN (2006) Comparative proteomic analysis provides new insights into chilling stress responses in rice. Mol Cell Proteom 5(3): 484-496.
- Prasad PVV, Pisipati SR, Momčilović I, Ristic Z (2011) Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EFTu expression in spring wheat. J Agron Crop Sci 197: 430-441.
- Akdemir H, Kaya E, Özden Y (2010) In vitro proliferation and minimum growth storage of fraser photinia: Influences of different medium, sugar combinations and culture vessels. Sci Hortic 126(2): 268-275.
- Michaletti A, Naghavi MR, Toorchi M, Zolla L, Rinalducci S (2018) Metabolomics and proteomics reveal drought-stress responses of leaf tissues from spring-wheat. Sci Rep 8(1): 1-18.
- Guldag S, Ozkaya DE, Kaya E (2023) Determination of genetic variations of post-micropropagated sweet orange (Citrus Sinensis (L.) Osbeck) micro-shoots by ISSR marker technique. Curr Invest Agric Curr Res 10(2): 1393-1398.
- Nemati N, Piro A, Norouzi M, Vaheda MM, Nisticò DM, et al. (2019) Comparative physiological and leaf proteomic analyses revealed the tolerant and sensitive traits to drought stress in two wheat parental lines and their F6 progenies. Environ Exp Bot 158: 223-237.
© 2023 © Kaya E. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






