- Submissions

Full Text
Environmental Analysis & Ecology Studies
Potential Ecological Risk due to Heavy Metal Pollution in Water Bodies
Singhal RK*
Analytical Chemistry Division, Bhabha Atomic Research Centre, India
*Corresponding author: Dr R.K.Singhal Analytical Chemistry Division Mod Lab, Bhabha Atomic Research Centre Trombay, Mumbai-400085, India
Submission: March 25, 2019;Published: May 28, 2019

ISSN 2578-0336 Volume5 Issue5
Abstract
Aquatic sediments can absorb metals to levels many times higher than the water column concentration, so it is considered a sink and reservoir of contaminants, such as metals. After a series of natural processes, the water borne metals would deposit in the sediment finally. Hence, the quantity of metals contained in the sediment reflects the degree of pollution for the water body. There were number of models purpose to evaluate the ecological risk due to accumulation of metals in sediment among others, work carried out by Muller is widely accepted internationally to evaluate the ecological risk.
Keywords:Aquatic sediments; Metals; Pollution; Ecological risk, Muller
Introduction
An ecological risk assessment is the process for evaluating how likely it is that the environment may be impacted as a result of exposure to one or more environmental stressors such as chemicals, and heavy metals etc. Heavy metal pollution is world-wide environmental problem. The concentration of trace metals in different water bodies get rise due to high inputs from natural, as well as anthropogenic sources. Thus, understanding the transport and distribution of trace metals in water bodies is an important goal of environmental chemists [1,2].
One of the most distinguishing features of metals from other toxic pollutants is that, they are not biodegradable. Many metals entering bodies of natural water can get incorporated and accumulated in the sediments after a series of natural processes, the water borne metals would deposit in the sediment finally. Hence, the quantity of metals contained in the sediment reflects the degree of pollution for the water body.
The favorable physicochemical conditions of the sediment can remobilize and release the metals back to the water column. There are many instances where specific local sources such as discharge from smelters (Cu, Pb, Ni), metal based industries (Zn, Cr and Cd from electroplating), paint and dye formulators (Cd, Cr, Cu, and Zn), petroleum refineries (As, Pb), as well as effluents from chemical manufacturing plants are some of the major contributor of metals into the water bodies. Once heavy metals enter into the environment, their potential toxicity is controlled to a large extent by their physicochemical form [2]. The total metal ion concentration in an aquatic environment is distributed between particulate and soluble forms.
Metals beyond a point are toxic to aquatic organisms and further they could threaten the aquatic ecology system [2,3]. Therefore, many studies effort has been directed toward the distribution of metals in aquatic. It is believed that organisms in different trophic levels are suffering from metal toxicities. Accumulation of heavy metals in organisms shows different tolerant capabilities among aquatic food webs and human health is under threats from exposure to heavy metals through seafood intake. There were number of models purpose to evaluate the ecological risk due to accumulation of metals in sediment and thereby their constant release in the aquatic environment and impacted the life of flora and fauna. Among many studies work carried out by Muller is widely accepted internationally to evaluate the ecological risk [3].
The geo-accumulation index (Igeo), Enrichment Factor (EF), and modified pollution index (MPI) were applied to estimate the degree of metals contamination. The Igeo values for the metals to be monitored can calculated using the Muller expression
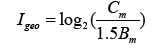
where Bm is the background content of metals in the earth’s crust
The EF is carried out by normalizing the metal concentration based on geological characteristics of the sediment. It is defined as follows
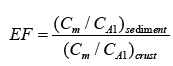
where Cm and CAl are the metals and Al content in sediments or in earth crust, respectively. Aluminum is a major metallic element found in the earth’s crust; its concentration is somewhat high in sediments and is not affected by man-made factors in general except area having bauxite depoit. Thus, Al has been widely used for normalizing the metal concentration in sediments. The MPI is calculated using the formula developed by Brady et al. [4]:
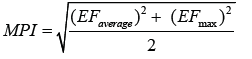
Where EFaverage and EFmax are average and max value, respectively, in all the EF of metals studied. The MPI is a combination of the Nemerow Pollution Index, and EF. The MPI can provide a qualitative assessment of site pollution with multiple metals [5]. The mean effect range median quotient (m-ERM-q) and potential ecological Risk Index (RI) were employed to assess the biological effects and potential ecological risk in sediments. The RI can be calculated from the
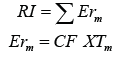
Where Erm is the potential ecological risk factor for metal, CF is the contamination factor, CF=Cm/Bm, Cm is the measure concentration of metals in sediment, Bm is the background concentration of metals, and Tm is the biological toxicity factor
The MPI is a comprehensive index that can be employed to conduct an overall assessment and comparison of the heavy metal contamination of different areas. The derivation of MPI is calculated from EFs, moreover the classification of MPI is also based on the EF thresholds as a basis to conduct the pollution level assessment [4]. The 6 MPI classes are: Class 0: unpolluted for MPI<1; Class 1: slight for 1≤MPI<2; Class 2: moderate for 2≤MPI<3; Class 3: moderate to heavy for 3≤MPI<5; Class 4: heavy for 5≤MPI<10; Class 5: severe for MPI≥10 [2].
The worked carried out by Hakanson proposes that potential ecological risk associated with metals in the surface sediments can be assessed by Erm and RI index [6]. Erm and RI that were proposed by him can be used to evaluate the potential risk of one metal and combination of multiple metals, respectively. The calculated Erm values can be categorized into five classes of potential ecological risks: low risk (Erm<40), moderate risk (40≤Erm<80), higher risk (80≤Erm<160), high risk (160≤Erm<320), and serious risk (Erm≥ 320). The calculated RI values can be categorized into four classes of potential ecological risks: low risk (RI<150), moderate risk (150≤RI<300), considerable risk (300≤RI<600) and very high risk (RI≥600).
References
- Singhal RK, Venkatesh M, Wagh DN, Basu H, Chavan T, Pimple MV, Reddy AVR (2012) Determination of chronological heavy metal deposition and pollution intensity in the bottom sediments of Mumbai Harbour Bay, India using 137Cs as tracer. Journal of Radioanalytical and Nuclear Chemistry 292(2): 863-869.
- Singhal RK, Preetha J, Karpe R, Tirumalesh K, Kumar SC, Hegde AG (2006) The use of ultra filtration in trace metal speciation studies in sea water. Environment International 32(2): 224-228.
- Müller G (1979) Schwermetalle in den sedimenten des rheinsveränderungen seit. Umschav 79: 133-149.
- Brady JP, Ayoko GA, Martens WN, Goonetilleke A (2015) Development of a hybrid pollution index for heavy metals in marine and estuarine sediments. Environ Monit Assess 187(5): 306.
- Nemerow NL (1991) Stream, Lake, Estuary, and Ocean Pollution, Van Nostrand Reinhold, New York, USA.
- Hakanson L (1980) An ecological risk index for aquatic pollution control a sedimentological approach. Water Res 14(8): 975-1001.
© 2019 Singhal RK. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






