- Submissions

Full Text
Environmental Analysis & Ecology Studies
Novel Adsorbent for Wastewater Treatment: Tailored Pore Size for Hazards from Aqueous Solution
Song Lou*
Department of Biological Systems Engineering, Virginia Polytechnic Institute and State University, USA
*Corresponding author:Song Lou, Department of Biological Systems Engineering, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061, USA
Submission: April 10, 2018; Published: May 24, 2018

ISSN 2578-0336 Volume2 Issue4
Abstract
Porous adsorption resin is one of the widely applied materials for removal of hazards from aqueous solution. The separation effect of resin is controlled by several interaction mechanisms, including sieving effect, electrostatic interactions and hydrogen bonds interaction. Resin pore size was reported to play an important role in the sieving effect of separation mechanism, which makes tailored pore size be considered as the main characteristic of next generation adsorbent. Here, we reviewed several widely used adsorption models and paid more attention to their potential values of calculating resin pore size for specialized target. We believe that this review is of certain significance and improvement of novel adsorption resins with tailored pore size. .
keywordsPorous adsorption resins, Tailored pore size, Adsorption models; Waste water treatment
Opinion
Porous adsorption resins possess many special characteristics, such as various categories, high mechanical strength, porous availability, high surface area, and long life times, which make it widely, used for separating targets and improve water quality [1-3]. As the title suggests, the important parameters of porous adsorption are pore structures and pore property. The IUPAC classification, in which pores are classified into macro- (>50nm), meso- (2-50nm), and micropores (< 2nm) is based on the different mechanisms occurring in these pores during N2 isothermal adsorption [4]. In order to gain a greater adsorption capacity and higher selectivity for toxic metallic species, chemical and physical modification is often undertaken to produce uniform adsorption resins by introducing selected functional groups into the matrix, which, in theory, modify the chemical composition of the surface of the adsorbents and hence improve their adsorption of target [5]. However, practical application by these modified resins is not much higher and their pore sizes result mainly from its single micro porous structure, which is unfavorable in adsorption application despite its greater surface area. Although many researchers are interested in enlarging the pore size of adsorption resins, research on the tailored pore size of adsorption onto resins lags severely. In several projects, the resins were modified to form more macro porous to increase adsorption capacity for wastewater treatment [6], but the various sizes of adsorbate were neglected. The chromium (VI) and arsenic (V) contamination in groundwater are major threat to human health in many regions of the world, however the efficient pore size of resins for these two hazards are different, which were reported to be mesopores for chromium VI [7] and micropores for arsenic V [8]. Thus, the adsorption resins with tailored pore size are eager in the field of wastewater treatment. Before selecting optimal pore size for adsorbate, the interaction mechanism between resins and hazards need to be investigated. Sieving effect play an important role in separation and enrichment of molecule by adsorbent. In view of their particular application, this effect is basically controlled by pore physical structure, especially pore size and particle diameter. In addition, it is postulated that the pore size dictated the adsorption behavior with resins containing related larger pores following Langmuir model whereas those containing smaller pores following Freundlich model [9]. The classical adsorption kinetics model, Langmuir kinetics model (Equation 1), assumes the surface of adsorbate is energetically homogeneous [10,11].
Figure 1:
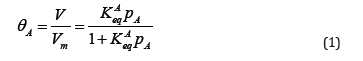
(pA is adsorbate’s partial pressure; V is the volume of adsorbate; θA is the fractional occupancy of the adsorption sites; Vm is the volume of the monolayer; Keq is the associated equilibrium constant). By contrast, the Langmuir-Freundlich model (Equation 2) is another equation, which can’t be solved analytically and considers the effect of surface heterogeneity [12-14]. However, the rate constant of this model can be obtained by an approximation method.
Figure 2:
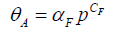
(θA is the fractional occupancy of the adsorption sites; αF and CF are fitting parameters). These two adsorption behavior models usually lead different adsorption kinetics properties since the larger pore result in a faster adsorption equilibrium, correspondingly. Besides the primary mechanism of sieving effect, the separation and enrichment of adsorbate also rely on physical force, such as hydrogen bonds and electrostatic interactions. However, the interaction between resins and targets is mostly driven by different interaction with the order of sieving effect >> electrostatic interactions > hydrogen bonds. Moreover, the interaction of physical force is controlled by resins matrix and adsorbate structure, and tailoring resin pore size according to adsorbate do not affect the adsorption mechanism of physical force. Thus, the next generation of adsorbent for wastewater treatment is the tailored pore size for hazards from aqueous solution.
The model of calculating required pore size appears to be more important for the novel strategy of tailored resins. There is less attention on the potential practice-guiding impact of related model. Corroll and Berens suggested a three-parameter, two-compartment model (Equation 3) accounting for the influence of sphere diameter [15]. This monophasic model always gives a good fit of data in each case, however the adsorption is not considered as rapid, slow and even slower procedures, but only a global process instead.
Figure 3:

(ϕr is fast adsorption rate coefficient; ar and as are the diameters of the spheres; Dr and Ds are diffusion coefficient). My project was performed in order to determine the adsorption profiles of adsorbate on resins [16,17]. According to the theory of the first-order, two-component four-parameter model, a new model [18] incorporating sphere size can be described by the following (Equation 4):
Figure 4:
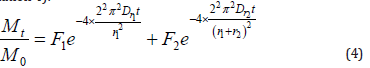
(Mt is the solid-phase sorbate concentration at a given time, M0 is the initial solid-phase adsorbate concentration, F1 and F2 are different compartments’ fractions, Dr is the diffusion coefficient, and r1 and r2 are the diameters of the spheres, on which the different compartments of adsorption process are mainly carried out). This model provided a good fit of adsorption data, and a sphere size parameter was considered to be included to supply guiding of tailored pore size. In addition, further adsorption steps could be considered into the analysis process and a sphere-size model in which the adsorption process contains more compartments was proposed as (Equation 5).
Figure 5:
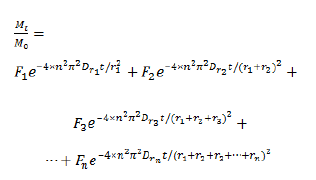
This model supplied parameters of sphere size, which could transformed to pore size according to BET results to reflects the effect of pore-size on adsorption kinetics process, and guides the selection or modification of porous adsorption resin for specialized target. Overall, the progress toward adsorption models with resin pore size is encouraging, and this is hope that further development of new calculating strategy of tailored pore size will help to bring forth better separation of targeted hazards from aqueous solution.
References
- Tratnyek PG, Johnson RL (2006) Nanotechnologies for environmental cleanup. Nano Today 1 (2): 44-48.
- Ma C, Tang J, Wang H, Tao G, Gu X, et al. (2009) Preparative purification of salidroside from Rhodiola rosea by two-step adsorption chromatography on resins. J Sep Sci 32(2): 185-191.
- Geng X, Ren P, Pi G, Shi R, Yuan Z, et al. (2009) High selective purification of flavonoids from natural plants based on polymeric adsorbent with hydrogen-bonding interaction. J Chromatogr A 1216(47): 8331-8338.
- Zdravkov BD, Cermak J, Sefara M, Janku J (2007) Pore classification in the characterization of porous materials: a perspective. Central European Journal of Chemistry 5(2): 385-395.
- Yang WB, Li A, Fan J, Yang L, Zhang Q (2006) Adsorption of branched alkylbenzene sulfonate onto styrene and acrylic ester resins. Chemosphere 64(6): 984-990.
- Liu Y, Liu J, Chen X, Liu Y, Di D (2010) Preparative separation and purification of lycopene from tomato skins extracts by macro porous adsorption resins. Food Chem 123(4): 1027-1034.
- Hokkanen S, Bhatnagar A, Repo E, Lou S, Sillanpää M, et al. (2016) Calcium hydroxyapatite microfibrillated cellulose composite as a potential adsorbent for the removal of Cr(VI) from aqueous solution. Chem Eng J 283: 445-452.
- Zhou Y, Yao J, He M, Choi MM, Feng L, et al. (2010) Reduction in toxicity of arsenic(III) to Halobacillus sp. Y35 by kaolin and their related adsorption studies. J Hazard Mater 176(1-3): 487-494.
- Idris SA, Alotaibi KM, Peshkur TA, Anderson P, Morris M, Gibson LT (2013) Adsorption kinetic study: effect of adsorbent pore size distribution on the rate of Cr (VI) uptake. Microporous Mesoporous Mater 165: 99-105.
- Nasernejad B, Zadeh TE, Pour BB, Bygi ME, Zamani A (2005) Camparison for biosorption modeling of heavy metals (Cr(III), Cu(II), Zn(II)) adsorption from wastewater by carrot residues. Process Biochem 40(3- 4): 1319-1322.
- Rengaraj S, Moon SH (2002) Kinetics of adsorption of Co (II) removal from water and wastewater by ion exchange resins. Water Res 36(7): 1783-1793.
- Raghunath S, Anand K, Gengan RM, Nayunigari MK, Maity A (2016) Sorption isotherms, kinetic and optimization process of amino acid proline based polymer nano composite for the removal of selected textile dyes from industrial waste water. J Photoch Photobio B 165: 189-201.
- Khorramfar S, Mahmoodi NM, Arami M, Gharanjig K (2010) Equilibrium and kinetic studies of the cationic dye removal capability of a novel biosorbent Tamarindus indica from textile waste water. Color Technol 126(5): 261-268.
- Al-Ghouti MA, Khraisheh MAM, Allen SJ, Ahmad MN (2003) The removal of dyes from textile wastewater: a study of the physical characteristics and adsorption mechanisms of diatomaceous earth. J Environ Manage 69(3): 229-238.
- Berens AR, Huvard GS (1981) Particle-size distribution of polymer powders by analysis of sorption kinetics. J Dispersion Sci Technol 2(2- 3): 359-378.
- Lou S, Chen ZB, Liu YF, Ye HL, Di DL (2012) Synthesis of functional adsorption resin and its adsorption properties in purification of flavonoids from hippophae rhamnoides L. Leaves. Industrial & Engineering Chemistry Research 51(6): 2682-2696.
- Lou S, Di D (2012) Synthesis of resins with ionic liquids for purification of flavonoids from Hippophae rhamnoides L. leaves. J Agric Food Chem 60(26): 6546-6558.
- Lou S, Liu YF, Bai QQ, Di DL (2012) Adsorption mechanism of macro porous adsorption resins. Prog Chem 24(8): 1427-1436.
© 2018 Song Lou. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






