- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Nanotechnology Based Drug Delivery Systems for Overcoming Blood Brain Barrier: A Mini Review
Anurag Yadav1* and Kusum Yadav2
1Department of Microbiology, India
2Department of Biochemistry, India
*Corresponding author:Anurag Yadav, Department of Microbiology, College of Basic Science and Humanities, Sardarkrushinagar Dantiwada Agricultural University, Gujarat, India
Submission: June 13, 2023;Published: July 19, 2023

ISSN: 2832-4439 Volume3 Issue2
Nanotechnology-enabled drug delivery systems have emerged as a promising tool for overcoming the Blood Brain Barrier (BBB) and delivering drugs to the Central Nervous System (CNS). This mini-review provides an overview of recent advancements in nanotechnology to improve blood-brain barrier penetration. It covers different approaches, such as using targeted ligands and receptors, engineered carriers and transporters, and surface modifications for targeting the blood-brain barrier. Polymeric nanoparticles, liposomes, and metallic nanoparticles, such as silver and zinc oxide, are discussed in the context of their unique properties and applications.
Preclinical and clinical advances in nanotechnology-based BBB delivery are discussed, including transcellular nanotechnology-based brain drug delivery and preclinical and clinical studies of nanocarriers for CNS disorders. Although nanotechnology has shown great potential for treating CNS diseases, several challenges remain. The major challenges and future perspectives for constructing brain-targeted delivery systems are also discussed, particularly limitations associated with the blood-brain barrier and clinical obstacles to CNS disease treatment. In conclusion, the development of nanotechnology-based drug delivery systems promises to revolutionize the treatment of CNS diseases.
Keywords:Nanoparticles; Blood brain barrier; Drug delivery systems; Engineered carriers
Abbreviations:BBBL: Blood Brain Barrier; CNS: Central Nervous System; NPs: Nanoparticles; ROS: Reactive Oxygen Species
Introduction
The Blood Brain Barrier (BBB) serves as a natural defense mechanism that impedes the delivery of therapeutic agents to the Central Nervous System (CNS). Nanoparticles (NPs) have emerged as a promising tool for targeted drug delivery across the BBB [1-6]. The size of NPs is in the range of 10-200nm, which allows them to readily cross the tightly packed endothelial cells of BBB compared to conventional drugs [1,2]. Several nanotechnology-based drug delivery systems have been designed to enhance CNS delivery [2-5]. Furthermore, NPs have improved drug delivery across the BBB by partially overcoming this obstacle, thus facilitating drug delivery [3,4]. Thus, engineered NPs with unique physicochemical properties can cross the BBB, which may be a promising strategy to solve biomedical and pharmacological problems in treating brain diseases such as Alzheimer’s disease and Parkinson’s disease [2,4,5].
Efficient and convenient drug-loading strategies are crucial for optimizing drug and NP interactions. While covalent bonding is a traditional method for drug loading that suffers from versatility and time consumption limitations, noncovalent adsorption is a frequently employed strategy due to its simplicity and rapid binding capabilities. Ligand modification of nanovehicles offers substantial advantages, including improved receptor reactivity and increased BBB permeability compared to unmodified nanovehicles [7]. For instance, the attachment of transferrin peptide to NPs enables effective surface dispersion, even with smaller particle sizes [8].
Moreover, amphipathic peptide modified NPs exhibit a high affinity for the BBB and remarkable stability [9]. NP-based formulations, such as polymeric NPs, lipid NPs, and dendrimers, have gained attention as potential carriers to enhance drug delivery across the BBB [10]. These formulations offer improved drug solubility, sustained release, and enhanced brain accumulation. Surface modification and functionalization techniques are employed to optimize the properties of NPs for crossing the BBB and improving drug delivery efficiency.
Nanotechnology Approaches to Enhance BBB Penetration
The BBB presents inherent challenges to traditional drug delivery systems that are thwarted in their attempts to deliver therapeutic agents to the brain. The BBB is a tightly packed endothelial cell layer surrounding the brain that restricts the entry of high-molecular-weight molecules into the brain [11]. Therefore, using nanocarriers or NPs to penetrate the BBB is immensely promising.
Carbohydrate-based NPs , nanogels, nanocerium, nanosilver, dendrimers, and gold NPs are unique platform options for molecular transport and targeted drug delivery, providing biocompatibility, biodegradability, and reduced toxic side effects [12,13] (Figure 1). Nanocarriers can help protect against oxidative stress caused by free radicals and amyloid-beta peptide oligomers, providing neuroprotection against toxicity [13]. Moreover, nanocarriers have become valuable tools for bioimaging and controlled drug delivery systems, addressing the challenges of delivering substances across the BBB [14].
Figure 1:Strategies for brain delivery of NPs.
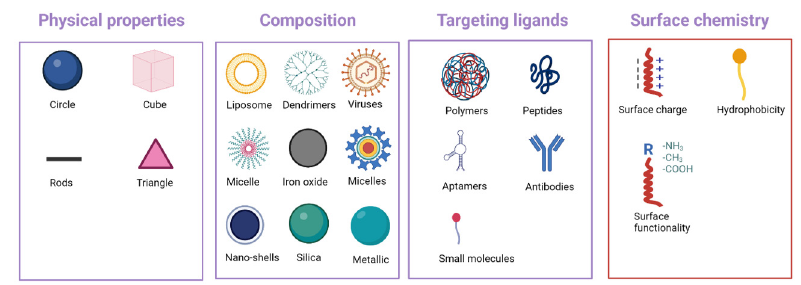
Multiple types of nanocarriers have been studied in treating CNS diseases, such as Alzheimer’s and Parkinson’s disease, and brain tumors [12,14]. Polymeric NPs, solid lipid NPs, nanostructured lipid carriers, microemulsions, nanoemulsions, and liquid crystals are promising for delivering nano formulations via various administration routes [15]. Lipid-based NPs qualify as the most established and effective drug delivery system regarding clinical translation [16]. Nanocarrier-based drug delivery systems seem to offer considerable promise for delivering drugs to the brain, particularly for neurodegenerative disorders [17]. Intranasal delivery of NPs also holds substantial promise as a viable approach for managing Alzheimer’s disease. This innovative strategy involves NPs systems that efficiently transport drugs to the brain, enabling targeted therapeutic interventions [17].
Targeted Ligands and Receptor-Mediated Transport across the BBB
The BBB can be targeted using three main categories of endogenous transportation: carrier-mediated transport, active efflux transport, and receptor-mediated transport [18] (Figure 2). The receptor-mediated transport across the BBB seems a practical approach for brain drug delivery [18,19]. Nevertheless, ligands play a significant role in facilitating receptor-mediated transport by binding with the proteins present in the cell [20]. The conjugation of ligands to the formulation enhances the targeting of the drug to the specific target location [18,19].
Figure 2:Various nanovehicle transport pathways through the BBB.
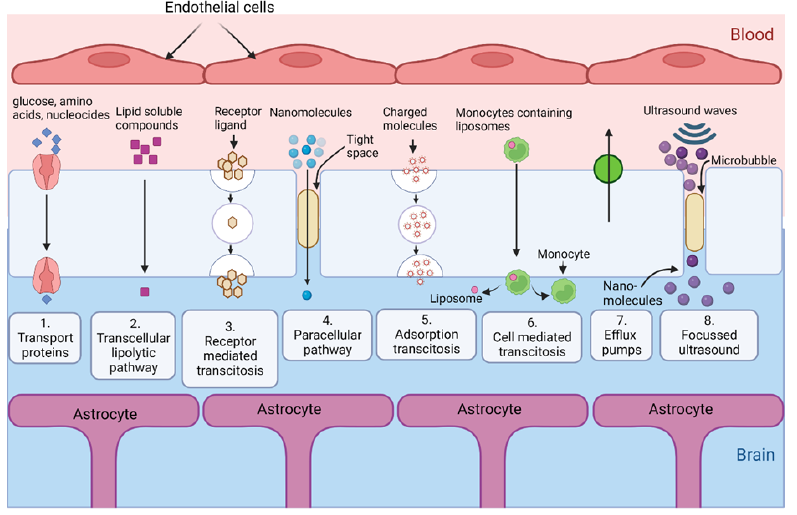
Several approaches using nanocarriers, such as liposomes, niosomes, micelles, and NPs with manifested surface modifications using either covalent or noncovalent methods to append suitable ligands, are being explored to achieve drug delivery to the brain [18,20,21]. The ligand-based NP-mediated targeted delivery of drugs can potentially reduce the dosage and optimize their release properties, increase specificity and bioavailability, improve shelf life, and decrease toxicity [21]. Several NP-based delivery systems, including lipid-based nanocarriers and carbon dots, have been developed that enable higher penetration through the BBB and maintain drug plasma levels in the desired range, owing to the control release profile [21]. Moreover, nanodrug carriers are being explored for nerve agent detoxification [22]. For example, transferrin-modified mesoporous silica NPs effectively penetrate the BBB and restore cerebral AChE activity via the released HI-6, preventing brain damage caused by toxic nerve agent poisoning [22].
Engineered Carriers and Transporters
Nanovehicles, a promising and innovative area of research for delivering medicine to the brain, have exceptional mechanical properties and can be adapted and tuned to easily transport across the BBB [23]. By manipulating physical characteristics such as surface charge, coating ligands, size, and shape, nanovehicles can significantly enhance transport efficiency, medication controllability, prevention of RES uptake, and therapeutic agent stability [24]. The size of nanovehicles is crucial for their intracellular localization and passage through the BBB, while the shape of particles, particularly spherical NPs, can impact cellular absorption of medications [23]. Additionally, the surface charge of nanovehicles affects their distribution in the body and their residence time in the bloodstream, with a negative or neutral charge reducing protein sticking and cellular absorption compared to a positive charge [23].
Engineered carriers and transporters, such as cell-based carriers, Trojan horse strategies, and receptor-mediated transport, offer innovative approaches to bypass the BBB [25]. Among these approaches, utilizing endogenous cells like stem cells and immune cells as carriers show great potential in delivering therapeutics across the BBB [25]. Transporter proteins, including glucose transporters and efflux pumps, also enhance drug transport across the BBB [25]. Moreover, researchers have developed dualtargeting liposomes decorated with APRPG and GRGDS peptides to simultaneously target VEGFR-1 and integrin αvβ3, resulting in enhanced binding to stimulated endothelial cells and increased tumor accumulation [26]. This dual-targeting approach improves the affinity of liposomes for target cells and holds promise for active-targeted drug delivery in cancer treatment. Transporter proteins, including glucose transporters and efflux pumps, enhance drug transport across the BBB. Nevertheless, nano vehicles have arisen as an exceptionally compelling and groundbreaking field of study in the delivery of brain medicine [27].
Surface Modification Strategies for BBB Targeting
The limited effectiveness of treating CNS diseases stems from the presence of the BBB, which acts as a restrictive barrier, and impeds the delivery of therapeutics to the brain. Recent advancements in nanotechnology offer potential solutions to overcome this obstacle. Among the strategies explored, one approach involves the surface modification of synthetic AuNPs with brain-targeted exosomes. Researchers demonstrated that this modification facilitates effective brain targeting, as evidenced by the enhanced penetration of the BBB [28]. The strategy exhibits excellent promise and could find applications in therapeutics and imaging. Another strategy entails the conjugation of nanodrugs with mesoporous silica NPs (MSNs) [22]. This approach has proven effective in treating acute chemical brain poisoning [22]. Using transferrin-modified MSNs, drugs can be delivered quickly and effectively through the BBB, especially for treating brain damage caused by organophosphate exposure. Nanodrug carriers have been investigated as a potential solution for brain tumor treatment [29]. Furthermore, these carriers possess superior drug transport capacity and easily control drug release properties [29]. As a result, they hold promise in overcoming the BBB and facilitating enhanced drug delivery for treating brain tumors. Pseudoephedrine hydrochloride, a watersoluble anticancer nanodrug compound, has shown potential for brain cancer treatment [30]. Researchers have suggested using synchrotron radiation to transport therapeutic anticancer nanodrugs across the BBB, thereby enabling effective treatment of brain diseases [31].
Nonetheless, nanotechnology offers promising solutions to overcome these challenges, allowing for targeted drug delivery to the brain. The NP-mediated targeted drug delivery presents a promising approach with numerous advantages. This method significantly reduces dosage requirements, optimizes drug release properties, enhances specificity and bioavailability, and reduces toxicity [21]. The development of multifunctional biomimetic nanodrugs exhibits considerable potential in overcoming limitations related to poor drug targeting, low BBB penetration, and short biological half-lives [32]. These nanodrugs offer a versatile platform for efficiently treating glioblastoma xenografts, capitalizing on their multifunctional properties [33]. Researchers have also focused on strategies targeting the transferrin receptor for treating glioblastoma [34]. In this regard, surface modification of NPs with transferrin, antibodies, and targeting peptides has shown promise [34]. In the context of depression treatment, researchers have synthesized CeO2@BSA nanoclusters as a novel nanodrug targeting Reactive Oxygen Species (ROS) [35]. This approach exhibits remarkable ROS scavenging, BBB penetration capacity, rapid metabolism, and negligible adverse effects in vitro and in vivo. It offers a promising therapeutic avenue for depression treatment. Lastly, a study investigated the therapeutic efficiency of a multifunctional hybrid nanostructure against glioblastoma [36]. The researchers found that dual-targeted NPs displayed enhanced uptake by glioblastoma cells, leading to an overall superior inhibitory effect compared to traditional drug delivery methods.
Preclinical and Clinical Advances in Nanotechnology-based BBB Delivery
Transcellular nanotechnology-based brain drug delivery
The transcellular route is a widely explored method for delivering therapeutics to the brain [37]. Incorporating anticancer agents in 100-300nm nanodevices allows their delivery in tissues with a fenestrated vasculature and reduced lymphatic drainage [38]. Moreover, developing highly-specific ligands and surface proteins has facilitated the engineering of nanocarriers for targeted drug delivery [39]. In CNS tumors, multifunctional NPs have brought revolutionary advances in targeted drug delivery [40]. Similarly, lipid-based nanocarriers have shown promising results in delivering drugs against major barriers, such as the skin and BBB [41].
Preclinical and clinical studies of Nanocarriers for CNS disorders
Recent preclinical and clinical studies have focused on using nanocarriers for CNS disorders. In Alzheimer’s disease, nanotechnology-based drug delivery approaches have been studied for their pathology, and various NPs are being developed for the same [42]. Nanotechnology-based approaches have been used in preclinical and clinical studies to address CNS drug delivery challenges in Parkinson’s disease [42]. Nanotechnologybased strategies have also been employed in ischemic stroke for therapeutic delivery to the brain [42]. Using multifunctional NPs in CNS tumors has brought revolutionary advances in targeted drug delivery [39]. Developing nanocarriers with particular ligands and surface proteins has facilitated targeted drug delivery to CNS tumors [39].
Challenges and Future Perspectives
While nano formulations have demonstrated effectiveness in improving drug delivery to the brain, further exploration of novel formulations is necessary [43]. Achieving optimal drug loading and release profiles is a key challenge in utilizing engineered carriers and transporters in nanotechnology-based drug delivery systems. Designing carriers that can encapsulate and release drugs in a controlled manner is crucial for maximizing therapeutic outcomes. It involves finding a delicate balance between stability and controlled release, which requires careful consideration of the physicochemical properties of the carrier materials. Another significant challenge is ensuring the biocompatibility and safety of nanocarriers. As drug delivery systems become more complex and diverse, assessing their potential toxicity and immunogenicity is essential. Nanocarriers must be designed to minimize adverse effects on healthy tissues and cells while still effectively delivering the therapeutic cargo. Rigorous preclinical and clinical studies are necessary to evaluate the safety profiles of these engineered carriers and transporters. Scalability and manufacturing processes for nanocarriers present another major challenge [44].
Transitioning promising nanotechnology-based drug delivery systems from the lab to large-scale production is complex. Developing cost-effective and scalable manufacturing methods while maintaining the integrity and quality of the carriers is crucial for the future commercialization and widespread use of these systems [44,45].
Despite these challenges, the future perspectives of nanotechnology-based drug delivery systems are highly promising. [46]. Engineered carriers can precisely target specific cells or tissues, opening up new possibilities for personalized medicine [44]. These systems can potentially enhance the therapeutic index of drugs, reduce side effects, and improve patient compliance. Furthermore, nanocarriers can be designed to overcome biological barriers, such as the BBB, enabling the delivery of therapeutics to previously inaccessible sites [44]. In addition to their therapeutic applications, nanotechnology-based drug delivery systems hold great potential for diagnostic purposes. Incorporating imaging agents or sensors into nanocarriers allows for real-time drug distribution, pharmacokinetics, and therapeutic response monitoring. This integration of diagnosis and treatment can revolutionize healthcare, enabling personalized and precise medicine [44].
Conclusion
Nanotechnology-based drug delivery systems are promising for treating and Managing Central Nervous System (CNS) disorders. These systems can overcome biological barriers such as the BBB, enabling the delivery of drugs to previously inaccessible regions of the body. Among the various nanotechnology-based drug delivery systems, NPs have emerged as a powerful tool for targeted drug delivery across the BBB. Polymeric NPs, lipid NPs, and dendrimers have been designed to enhance CNS delivery, and surface modification strategies have been investigated to increase BBB permeability. Targeted ligands, receptor-mediated transport across the BBB, and transporter proteins have been explored to improve drug transport. When developing nanocarriers, it is crucial to ensure optimal drug loading and release profiles and biocompatibility and safety. Clinical studies have shown that nanocarriers hold promise for treating CNS disorders such as Alzheimer’s disease, Parkinson’s disease, and CNS tumors. However, further exploration of novel formulations and rigorous preclinical and clinical studies are needed to evaluate the safety and efficacy of these engineered carriers and transporters. Overall, the future of nanotechnology-based drug delivery systems is promising, and they show remarkable potential for personalized medicine. With continued research and development, nanocarriers could provide effective and safe treatments for CNS disorders, allowing us to overcome the biological barriers of the brain and deliver targeted drugs to the affected areas.
References
- Bhaskar S, Tian F, Stoeger T, Kreyling W, Fuente JM, et al. (2010) Multifunctional nanocarriers for diagnostics, drug delivery and targeted treatment across blood-brain barrier: perspectives on tracking and neuroimaging. Part Fibre Toxicol 7(3):
- Kundu P, Das M, Tripathy K, Sahoo SK (2016) Delivery of dual drug loaded lipid-based nanoparticles across the blood-brain barrier impart enhanced neuroprotection in a rotenone induced mouse model of parkinson's disease. ACS Chem Neurosci 7(12): 1658-1670.
- Aslund AKO, Berg S, Hak S, Morch Y, Torp SH, et al. (2015) Nanoparticle delivery to the brain-By focused ultrasound and self-assembled nanoparticle-stabilized microbubbles. J Control Release 220(Pt A): 287-294.
- Shakeri S, Ashrafizadeh M, Zarrabi A, Roghanian R, Afshar EG, et al. (2020) Multifunctional polymeric nanoplatforms for brain diseases diagnosis, therapy and theranostics. Biomedicines 8(1): 13.
- Khan NH, Mir M, Ngowi EE, Zafar U, Khakwani M, et al. (2021) Nanomedicine: A promising way to manage Alzheimer's disease. Front Bioeng Biotechnol 9.
- Kendre PN, Kayande DR, Jain SP, Malge TG, Zadpe NN, et al. (2023) Polymeric nanoparticles: prospective on the synthesis, characterization and applications in nose-to-brain drug delivery. Current Nanoscience 19(5): 663-676.
- Miao YB, Zhao W, Renchi G, Gong Y, Shi Y (2023) Customizing delivery nano-vehicles for precise brain tumor therapy. J Nanobiotechnology 21(1): 32.
- Ferris DP, Lu J, Gothard C, Yanes R, Thomas CR, et al. (2011) Synthesis of biomolecule‐modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small 7(13): 1816-1826.
- Dixit S, Novak T, Miller K, Zhu Y, Kenney ME, et al. (2015) Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 7(5): 1782-1790.
- Ding S, Khan AI, Cai X, Song Y, Lyu Z, et al. (2020) Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater Today 37: 112-125.
- Poudel P, Park S (2022) Recent advances in the treatment of Alzheimer’s disease using nanoparticle-based drug delivery systems. Pharmaceutics 14(4): 835.
- Alajangi HK, Kaur M, Sharma A, Rana S, Thakur S, et al. (2022) Blood-brain barrier: Emerging trends on transport models and new-age strategies for therapeutics intervention against neurological disorders. Mol Brain 15(1): 1-28.
- Silant'ev VE, Shmelev ME, Belousov AS, Patlay AA, Shatilov RA (2023) How to develop drug delivery system based on carbohydrate nanoparticles targeted to brain tumors. Polymers 15(11): 5346.
- Abdelwahab GM, Mira A, Cheng YB, Abdelaziz TA, Lahloub MFI, et al. (2021) Acetylcholine esterase inhibitory activity of green synthesized nanosilver by naphthopyrones isolated from marine-derived Aspergillus niger. Plos One 16(9):
- Unnisa A, Greig NH, Kamal MA (2023) Nanotechnology: A promising targeted drug delivery system for brain tumours and alzheimer's disease. Curr Med Chem 30(3): 255-270.
- Fonseca-Santos B, Gremião MPD, Chorilli M (2015) Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomedicine 10: 4981-5003.
- Loh JS, Tan LKS, Lee WL, Ming LC, How C, et al. (2021) Do lipid-based nanoparticles hold promise for advancing the clinical translation of anticancer alkaloids? Cancers (Basel) 13(21): 5346.
- Bahadur S, Sachan N, Harwansh RK, Deshmukh R (2020) Nanoparticlized system: promising approach for the management of alzheimer's disease through intranasal delivery. Curr Pharm Des 26(12): 1331-1344.
- Jain A, Jain SK (2015) Ligand-Appended BBB-Targeted Nanocarriers (LABTNs) Crit Rev Ther Drug Carrier Syst 32(2): 149-180.
- Suarez VM, Vispo NS, Ramos OS (2021) Application of the phage display technology for the development of peptide- mediated drug delivery systems through the blood-brain barrier. Curr Pharm Biotechnol 22(11): 1394-1403.
- Mittal S, Ashhar MU, Qizilbash FF, Qamar Z, Narang JK, et al. (2020) Ligand conjugated targeted nanotherapeutics for treatment of neurological disorders. Curr Pharm Des 26(19): 2291-2305.
- Cerna T, Stiborova M, Adam V, Kizek R, Eckschlager T (2016) Nanocarrier drugs in the treatment of brain tumors. Journal of Cancer Metastasis and Treatment 2(10):
- Yang J, Fan L, Wang F, Luo Y, Sui X, et al. (2016) Rapid-releasing of HI-6 via brain-targeted mesoporous silica nanoparticles for nerve agent detoxification. Nanoscale 8(18): 9537-9547.
- Betzer O, Shilo M, Opochinsky R, Barnoy E, Motiei M, et al. (2017) The effect of nanoparticle size on the ability to cross the blood-brain barrier: an in vivo Nanomedicine 12(13) 1533-1546.
- Chellammal HSJ, Singh GKS, Sukumaran SK, Ramachandran D (2022) Phytonanoparticles for the treatment of Alzheimer’s disease: A review. Journal of Phytonanotechnology and Pharmaceutical Sciences 2(3): 1-6.
- Pardridge WM (2012) Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab 32(11): 1959-1972.
- Sugiyama T, Asai T, Nedachi YM, Katanasaka Y, Shimizu K, et al. (2013) Enhanced active targeting via cooperative binding of ligands on liposomes to target receptors. Plos One 8(6): e67550.
- Shadab, Mustafa G, Baboota S, Ali J (2015) Nanoneurotherapeutics approach intended for direct nose to brain delivery. Drug Dev Ind Pharm 41(12): 1922-1934.
- Khongkow M, Yata T, Boonrungsiman S, Ruktanonchai UR, Graham D, et al. (2019) Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood-brain barrier penetration. Sci Rep 9(1): 8278.
- Qiu Z, Yu Z, Xu T, Wang L, Meng N, et al. (2022) Novel nano-drug delivery system for brain tumor treatment. Cells 11(23): 3761.
- Bhaskaran NA, Kumar L (2022) Solid lipid nanoparticles-based drug delivery for targeting brain tumors. In Nanocarriers for Drug-Targeting Brain Tumors, pp. 237-268.
- Heidari A (2017) Transport therapeutic active targeting of human brain tumors enables anti-cancer nanodrugs delivery across the Blood Brain Barrier (BBB) to treat brain diseases using nanoparticles and nanocarriers under synchrotron radiation. Journal of Pharmacy and Pharmaceutics 4(2): 151-155.
- Yao Z, Jiang X, Yao H, Wu Y, Zhang F, et al. (2022) Efficiently targeted therapy of glioblastoma xenograft via multifunctional biomimetic nanodrugs. Biomater Res 26(1): 71.
- Zou Y, Liu Y, Yang Z, Zhang D, Lu Y, et al. (2018) Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Advanced Materials 30(51): 1803717.
- Ramalho MJ, Loureiro JA, Coelho MAN, Pereira MC (2022) Transferrin receptor-targeted nanocarriers: Overcoming barriers to treat glioblastoma pharmaceutics. Pharmaceutics 14(2): 279.
- Fu S, Chen H, Yang W, Xia X, Zhao S, et al. (2022) ROS-targeted depression therapy via bsa-incubated ceria nanoclusters. Nano Lett 22(11): 4519-4527.
- Ou Z, Li X, You Y, Liu D, Wang J (2023) Interpreting the therapeutic efficiency of multifunctional hybrid nanostructure against glioblastoma. Acs Omega 8(13): 12259-12267.
- Pawar B, Vasdev N, Gupta T, Mhatre M, More A, et al. (2022) Current update on transcellular brain drug delivery. Pharmaceutics 14(12): 2719.
- Caraglia M, Rosa D, Salzano G, Santini D, Lamberti M, et al. (2012) Nanotech revolution for the anti-cancer drug delivery through blood-brain barrier. Curr Cancer Drug Targets 12(3): 186-196.
- Meng J, Agrahari V, Youm I (2017) Advances in targeted drug delivery approaches for the central nervous system tumors: the inspiration of nanobiotechnology. J Neuroimmune Pharmacol 12(1): 84-98.
- Zhao X, Ye Y, Ge S, Sun P, Yu P (2020) Cellular and molecular targeted drug delivery in central nervous system cancers: advances in targeting strategies. Curr Top Med Chem 20(30): 2762-2776.
- Khan MS, Mohapatra S, Gupta V, Ali A, Naseef PP, et al. (2023) Potential of Lipid-based nanocarriers against two major barriers to drug delivery-skin and blood-brain barrier. Membranes (Basel) 13(3).
- Sa P, Singh P, Dilnawaz F, Sahoo SK (2022) Application of therapeutic nanoplatforms as a potential candidate for the treatment of CNS disorders: challenges and possibilities. Curr Pharm Des 28(33): 2742-2757.
- Nair SC, Thayyilakandy S, Arjun KK, Krishnakumar G, Gayathri PS (2019) A futuristic perspective in subsiding the symptoms of Parkinson’s Disease. International Journal of Research in Pharmaceutical Sciences 10(2): 975-989.
- Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, et al. (2019) Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res 23: 20.
- Werengowska CK, Wiśniewski M, Terzyk AP, Furmaniak S (2015) The chemistry of bioconjugation in nanoparticles-based drug delivery system. Advances in Condensed Matter Physics 1: 27.
© 2023 Anurag Yadav. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






