- Submissions

Full Text
Determinations in Nanomedicine & Nanotechnology
Cobalt Nanostructured Coatings for Methanol Anodic Oxidizing
Maryna Ved*, Nikolay Sakhnenko and Tatiana Nenastina
National Technical University, Ukraine
*Corresponding author: Maryna Ved, National Technical University, Ukraine
Submission: September 13, 2019;Published: November 21, 2019

ISSN: 2832-4439 Volume1 Issue3
Abstract
A. Co-Mo-W(Zr) electrolytic alloys of both amorphous & crystalline structure were deposited in pulse
mode.
B. Above alloys exhibit catalytic activity in methanol electrooxidizing in alkali media.
C. Ternary coatings were shown to be may be cycled and thus utilized as electrode materials for fuel cells.
Introduction
Eco-friendly Fuel Cells (FC), Solar Cells (SC) are among the promising renewable energy sources, however, the high cost of the noble metal electrodes prevents their dissemination and widespread use. Development of FC, SC and various Red-ox Flow Batteries (RFB) needs to create effective catalytic electrodes based on the transition metals. Among the most important requirements to electrode materials are chemical stability of the surface and inactivity to technological environment components; wide window of polarization potentials, in which electrode stays inactive; high selectivity and catalytic activity toward main electrode reactions; significant specific surface area. Even brief review gives an impression that in scientific literature for the last years there are too few publications on electrode materials on the basis of hi-tech materials, such as nanostructured and nanocrystalline materials based on the corrosion resistant amorphous metal alloys (metal glass), or nanostructured deposits by synergistic alloys [1-3]. The most efficient directions of catalytic materials synthesis are electrochemical technologies that provide flexibly control the composition, the deposition rate, the state of the surface, by varying the electrolyte nature and polarization mode [4-6]. Because of this it is possible to fabricate the deposits with desirable functional properties (synergistic or additive) [7-10].
Methods
Alloys Co-Mo-W(Zr) were deposited onto the steel substrate from a citrate-diphosphate bath in pulse mode [7,9]. The chemical composition of the coatings was determined by energy dispersive X-ray spectroscopy (EDS) on an Oxford INCA Energy 350 electron probe microanalysis integrated into the system of the SEM. The surface morphology of the deposits was studied with a Zeiss EVO 40XVP scanning electron microscope (SEM). The surface roughness was evaluated by the contact method on 10×10×2mm samples with an NT-206 scanning probe AFM microscope (CSC cantilever B as probe, probe tip radius 10nm). The AFM results are reflected at the Figure 1. The structure of the deposits was examined by X-ray diffraction analysis using a diffractometer (DRON-2.0) in the emission of iron anode and CuKα radiation. Electro catalytic properties of coatings were studied in model reaction of methanol electrooxidizing in alkali medium using Cyclic Voltammetry (CVA) technique [1].
Result and Discussion
Structure of the ternary alloys was found to be amorphous-crystalline (Figure 1), and coherent-scattering region size was of 2-8nm. Co-Mo-W coatings contain intermetallic phases Co7W6, and Co7Mo3 (Figure 2a), and Co3Mo and Co7Mo6 ones are found in the structure of Co-Mo-Zr deposits (Figure 2b). Analysis CVA obtained at the Co-Mo-Zr coated electrode polarization in methanol containing alkaline solution shows the ratio of reverse and direct current to be less than 1, and the direct peak changes more noticeably than the reverse one (Table 1), which indicates methanol adsorption on the electrode surface. There is not any significant change in potentials and peak currents after the eighth cycle of polarization.
Figure 1: Topography of the surface of alloy
A. Со-Mo-W and
B. Co-Mo-Zr
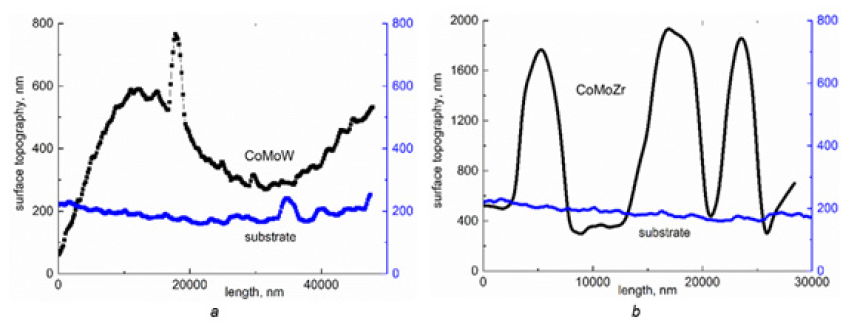
Figure 2:X-ray diffraction patterns for electrolytic alloys
a. Co-Mo-W, at. %: 1-Co-75, Mo-16, W-9; 2-Co-85, Mo-9, W-5.
b. Co-Mo-Zr, at. %: 1-Co-72.2, Mo-24.1, Zr-3.7; 2-72.9, Mo-24.9, Zr-2.2.
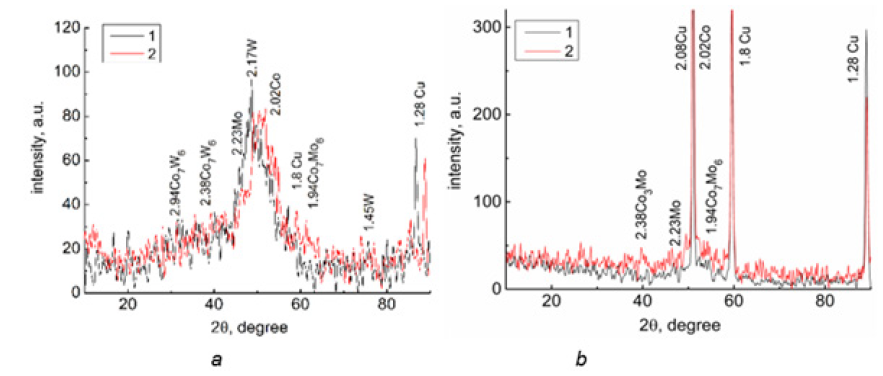
Table 1: Current of methanol oxidizing at Со-Mo-Zr coated electrodes vs cycle number.
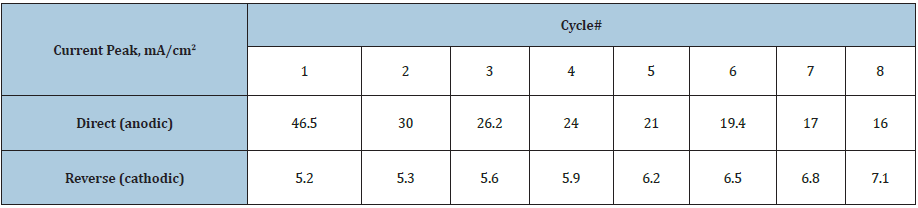
The results obtained indicate a rather high electrocatalytic activity of Co-Mo-Zr deposits, and it can be concluded that the (CH3OH)S → (HCHO)S reaction is the slow stage of net process, which allows for cycling latter and prevents the formation of carbon dioxide.
Conclusion
The activity of electrodes with ternary coatings in the oxidation of methanol is significantly higher than that of platinum, and for the Co-Mo-Zr alloy, the peak height is 2-2.5 times than for Co-Mo-W. The increased catalytic activity of the coatings is due to both a high degree of surface development and the synergistic effect of alloying metals.
References
- Vernickaite E, Tsyntsaru N, Cesiulis H (2016) Electrodeposited Co-W alloys and their prospects as effective anode for methanol oxidation in acidic media. Surf & Coat Tech 307: 1322-
- Kublanovsky V, Yapontseva Yu (2014) Electrocatalytic properties of Co-Mo Alloys electrodeposited from a citrate-pyrophosphate electrolyte. Electrocatalysis 5(4): 372-378.
- Mukhamedova G, Ved M, Sakhnenko N (2017) Appl Surf Sci 421: 68-76.
- Karakurkchi M, Ved I, Ermolenko (2016) Surf Eng Appl Electrochem 52: 43-49.
- Tsyntsaru N, Cesiulis H, Pellicer E (2013) Electrochim Acta 104: 94-103.
- Ahmad J, Asami K, Takeuchi A (2003) High strength Ni-Fe-W and Ni-Fe-W-P alloys produced by electrodeposition. Materials Transactions 44(10): 1942-1947.
- Ved M, Sakhnenko N, Koziar M, Slavkova MA (2016) Functional properties of electrolytic alloys of cobalt with molybdenum and zirconium. Functional Materials 23(3): 420-426.
- Schlesinger M, Paunovic M (2010) Modern Electroplating. In: Hoboken NJ, et al. (Eds.), (5th edn), USA.
- Sakhnenko N, Ved M, Hapon Yu (2015) Russ J Appl Chem 87: 1941-
- Pustovalov E, Modin E, Voitenko O, Aleksander NF, Aleksander V, et al. (2014) Structure relaxation and crystallization of the CoW-CoNiW-NiW electrodeposited alloys. Nanoscale Res Lett 9: 66.
© 2019 Maryna Ved. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






