- Submissions

Full Text
Developments in Clinical & Medical Pathology
Food Fortified with Carbohydrate-Fermenting Gut Microbes Producing Butyrate May Reverse Type 2 Diabetes Mellitus: A Silver Lining for Diabetes Research
Borkar SG*
Borkar’s Laboratory and Research Centre, India
*Corresponding author:Borkar SG, Borkar’s Laboratory and Research Centre, 301, Prestige Point building, In front of Nashik Road police station, Nashik- 422 101, Maharashtra, India
Submission: March 19, 2025;Published: May 29, 2025

ISSN:2690-9731 Volume2 Issue3
Abstract
Diabetes mellitus is a metabolic non-communicable disease affecting people of all ethnic groups around the world. To date, no cure for the disease is available except for its management by medication. It is a disease probably caused by changes in food habits, which alter the gut microbiome and certain bacterial species, their densities, and metabolic functions in the altered gut environment. Carbohydrate fermenting microbes producing butyrate may reverse Type-2 diabetes mellitus, as butyrate production in the gut environment regulates the blood glucose level and glucose intolerance. Species of Faecalibacterium, Eubacterium, Clostridium, Ruminococcus, and Roseburia are important carbohydrate-fermenting bacteria that are mostly involved in starch fermentation with the production of butyrate in the gut environment. Butyrates help to produce gut hormones that regulate blood sugar levels, which may improve insulin resistance. These bacterial species can be used to fortify the food material recommended for people with type 2 diabetes mellitus. A further research and formulation of such fortified food will be a silver lining for diabetes research. Available research suggests that certain foods, especially dry fruits and vegetables, are fortified with certain gut microbes, but not with the carbohydrate-fermenting butyrate-producing gut microbes, and therefore, it is of utmost importance for gut microbial diabetic research.
Keywords: Diabetes; Ethnic group; Gut microbiome; Butyrate; Fortified food
Introduction
Type 2 Diabetes Mellitus (T2DM) is one of the most common metabolic diseases in the world. Due to the rise in morbidity and mortality, it has become a global health problem. To date, T2DM still cannot be cured, and its intervention measures mainly focus on glucose control, as well as the prevention and treatment of related complications [1]. In type 2 diabetes, which affects approximately 463 million people worldwide, a person’s body gradually loses its ability to regulate blood sugar [2] effectively. Although prior research has connected changes in the gut microbiome to type 2 diabetes [3,4], a diverse large-scale study at a global level has been lacking. Mai et al. [5] analyzed data from the newly established microbiome, and cardiometabolic disease consortium (Micro-Cardio), wherein the dataset included genomic information from the gut microbiomes of 8117 people who had type 2 diabetes, pre-diabetes, or normal blood glucose levels, and were ethnically and geographically diverse, hailing from the United States, Israel, Sweden, Finland, Denmark, Germany, France, and China. They found a consistent set of microbial species that were linked to type 2 diabetes across their study population, including many that had never been reported before. To understand the role of these microbes in the gut, the researchers analyzed the species functional abilities. They found that certain strains of microbes had functions that may be linked to varied type 2 diabetes risk. For example, a strain of Prevotella copri- a common gut microbe that has the capacity to produce large quantities of branches-chain amino acids was more commonly seen in the gut microbiomes of people with type 2 diabetes. Previous studies have shown that people with chronically high levels of branched-chain amino acids in their blood have a higher risk of obesity and type 2 diabetes [6]. The researcher believed that changes in the gut microbiome cause type 2 diabetes.
Zhou et.al [1] reported that gut microbiota plays an important role in the development of metabolic diseases, especially T2DM [7] also opinioned that changes in gut microbiome may increase type 2 diabetes risk. It was found that specific species and strains of gut microbes were more common in people with type 2 diabetes. Bacteroides and Bifidobacterium are beneficial genera that are frequently reported in studies of type 2 diabetes. Roseburia and Faecalibacterium are found in lower frequencies in Type 2 Diabetes groups than in healthy control suggesting that their lower frequencies may be responsible for uncontrol metabolism associated with glucose regulation. According to a study by Candela et.al [8] type 2 diabetes patients had more Short-Chain-Fatty- Acid producers like Faecalibacterium, Roseburia, Lachnospira, Bacteroides, and Akkermansia in their gut microbiomes than the healthy individual who followed two different diet patterns (high fiber diet vs control diet). Zheng Li et al. [9] concluded that there is clear and growing evidence of a close relationship between the microbiota and diabetes, and this is worthy of future investments and research efforts. Dysbiosis in gut microbial composition and function is linked to immune responses and the development of metabolic diseases, including diabetes mellitus [9].
The composition of the gut microbiome may affect the likelihood of developing type 2 diabetes, according to the systematic review of Slouha et.al [10]. The changes to the microbiome may happen first, and diabetes develops later, not the other way around [5], and that prospective or interventional studies are needed to confirm this relationship. Research over the past decade has linked changes in the gut microbiome to the development of type 2 diabetes but has not been able to draw significant conclusions because of those studies’ small size and varied design. The gut microbiome’s relationship to complex, chronic, heterogeneous diseases like type 2 diabetes is quite subtle. The microbiome is highly variable across different geographic locations, and racial and ethnic groups. The study of a small, homogeneous population will probably give inconclusive results. Therefore, large and diverse populations are necessary for detailed microbiome variation studies. If further research confirms that these changes indeed contribute to the development of type 2 diabetes, researchers could use that knowledge to try to manipulate the microbiome to reduce type 2 diabetes risk.
Research Needs
Gut microbiome studies (of diabetes and non-diabetes) of all the ethnic groups around the world
There are 26 Ethnic groups reported around the world [11]. People who have a common language, race, religion, or cultural background are considered to be an ethnic group. These are the area-specific people’s groups, whose culinary and dietary practices differ from each other. It is apparent from Table 1 that irrespective of the variation in culinary and diet, the people from all these 26 Ethnic groups have diabetes. It means that it is not the diet that causes diabetes, but a change in diet and subsequent shift in the gut microbiome may be the reason for causing diabetes mellitus. Previous report from the metagenomics of the Human Intestinal Tract (MetaHIT) project indicated 32 core bacterial species in more than 80% of the European population that belonged to the genera Faecalibacterium, Roseburia, Bacteroides, Dorea, Clostridium, Eubacterium, Coprococcus, Ruminococcus, Alistipes, Collincella, Parabacteroides and Bifidobacterium [40]. In this study, Faecalibacterium, Eubacterium, Clostridium, Blautia, Ruminococcus and Roseburia were found to be core gut bacterial genera across the representative population of the world [41] (both rural and urban).
Table 1:Ethnic groups, their geographical locations, food habits, and major non-communicable diseases for consideration of gut microbiome studies.
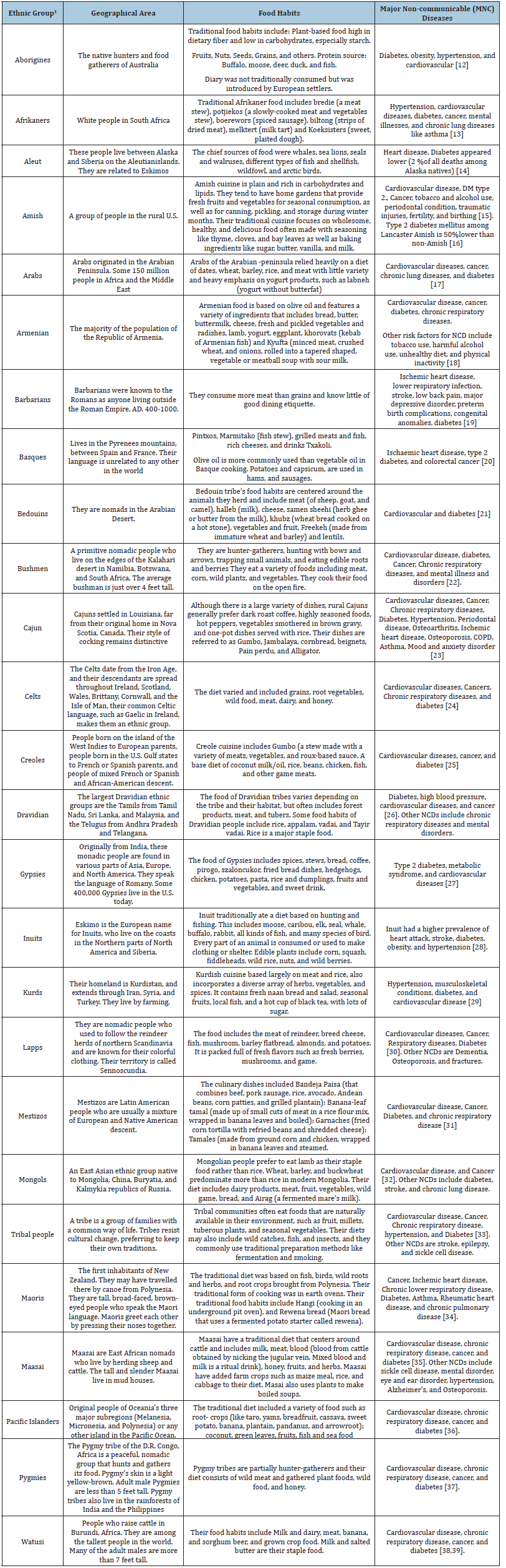
Further study of the gut microbiome may need to assess the presence of gram-negative bacteria [30] in these ethnic groups with diabetes and non-diabetes. Gram-negative bacteria may produce gut microbial variables such as bacterial Lipopolysaccharides (LPS) [42]. LPS is a toxin that induces insulin resistance, Type 2 diabetes, and Alzheimer’s disease. Plasma LPS may need to be measured in these ethnic groups to reduce the risk of Type 2 diabetes.
The bacterial species, particularly Faecalibacterium, Eubacterium, Clostridium, Ruminococcus, and Roseburia are important carbohydrate-fermenting bacteria that are mostly involved in starch fermentation with the production of butyrate. Butyrate is a Short-Chain Fatty Acid (SCFA) produced by gut microbiota that plays a key role in gut health with various functions. Butyrate is the primary energy source for colonocytes. It strengthens the gut barrier by targeting tight junctions, the mucus layer, and the production of antimicrobial peptides. It inhibits inflammation and oxidative stress. It can protect against epithelial injury and improve tissue repair. It promotes a healthy microbiome. It regulates metabolism and helps with the transepithelial transport of fluids. It helps to prevent non-alcoholic fatty liver disease, nonalcoholic steatohepatitis, inflammation, cancer, and liver injuries. Butyrates help to produce gut hormones that regulate blood sugar levels, which may improve insulin resistance and obesity. Butyrate is produced from fermentative carbohydrates of plant-origin food material, such as fibers, cellulose, and complex sugar in the colon with the help of gut bacteria [43]. Therefore, the production of butyrate in the gut by the gut microbiome seems to be the most important aspect in the regulation of blood sugar levels and needs further confirmation.
It will be advisable to study the gut microbiome of all the ethnic groups (diabetes and non-diabetes) to assess the presence/absence of carbohydrate-fermenting gut bacteria with their population density variation among diabetes and non-diabetes. This will give a clear-cut picture of gut microbial variables in these people. Once it is confirmed that carbohydrate fermenting bacteria and butyrate produced by these have a role in the regulation of blood sugar and diabetes, these can be used in the form of probiotics in the management and probably reversal of diabetes mellitus all over the world.
Furthermore, the performance of probiotic bacterial strains is influenced by the carrier food and its functional components, which while buffering the probiotic through the gastrointestinal tract, contribute to an efficient implantation of bacterial cells and regulate probiotic features [44]. Plant-based matrices are eligible substrates for hosting and delivering microbial populations because of their richness in nutrients, fibers, vitamins, minerals, and dietary bioactive phytochemicals. The health-promoting properties of solid plant-based matrices (particularly artichokes, table olives, apples, and cabbage) and their association with probiotic bacteria are indicative of the role of the food matrix in sustaining probiotic cells during product processing, digestive process, gut implantation, and finally in exerting beneficial effects [2,3]. The functional attributes of plant-based matrices, their structure and their suitability to fermentation make them appropriate for carrying probiotic strains that would take advantage of the characteristics of plant-based matrices and, by exploiting prebiotic and bioactive molecules, take benefit for their survival during product processing and shelf life as well as in the digestive process and gut colonization [45]. The available research on fortified food items with probiotics suggests that carbohydrate-fermenting probiotics producing butyrate can also be used in food fortification and diabetic reversal programs [46].
Conclusion
Diabetes mellitus, widely prevalent around the world in people of all ethnic groups, is probably caused by a change in carbohydrate metabolism, as a result of various factors, including changes in food habits. This change in food habits alters the human gut microflora responsible for carbohydrate metabolism. The involvement of certain gut microbial species related to carbohydrate metabolism plays an important role in the disease initiation and prevalence. These bacterial species, as probiotics, can alter carbohydrate metabolism and thereby diabetes. The food fortified with these carbohydrate-fermenting, butyrate-producing bacteria can be of significant importance in reversing type 2 diabetes.
References
- Zheng Z, Bao S, Dongsheng Y, Chunsheng Z (2022) Gut microbiota: An Important player in type 2 diabetes mellitus. Front Cell Infect Microbiol 12: 834485.
- Borkar SG (2023) Now, we need to breed the cereal food crop varieties with low glycemic index for 463 million diabetic population of the world. Biomedical Journal of Scientific & Technical Research 49(1): 40358-40364.
- Borkar SG (2023) Human gut microbes assisted agricultural food production for better human health: A new concept. Biomedical Journal of Scientific & Technical Research 48(3): 39728-39749.
- Natalia GV, Theodora S, Stylianos T (2018) Microbiome and diabetes: Where are we now. Diabetes Research Clinical Practice 146: 111-118.
- Mei Z, Wang F, Amrisha B, Danyue D, Dong DW, et al. (2024) Strain-specific gut microbial signatures in type 2 diabetes identified in a cross-cohort analysis of 8,117 metagenomes. Nature Medicine 30(8): 2265-2276.
- Cuomo P, Capparelli R, Iannelli A, Iannelli D (2022) Role of branched-chain amino acid metabolism in type 2 diabetes, obesity, cardiovascular disease and non-alcoholic fatty liver disease. International J Mol Sci 23(8): 4325.
- Welsh J (2024) Changes to Gut microbiome may increase type 2 diabetes risk. Harvard Medical School, USA.
- Candela M, Soverini M, Quercia S, Severgnini M, Peano C, et al. (2016) Modulation of gut microbiota dysbiosis in type 2 diabetic patients by macrobiotic Ma-Pi 2 diet. Br J Nutr 116(1): 80-93.
- Wei ZL, Kyle S, Jun JY, Lei Z (2020) Gut microbiota and diabetes: From correlation to causality and mechanism. World J Diabetes 11(7): 293-308.
- Ethan S, Atbeen R, Kiana F, Andrew G, Lucy AC, et al. (2023) Type-2 Diabetes mellitus and the gut microbiota: Systematic Review. Cureus 15(11): 49740.
- (2024) Fact Monster (2000-2017) Sandbox Networks, Inc., South Korea.
- Hotez PJ (2014) Aboriginal populations and their neglected tropical diseases. PLOS Neglected Tropical Diseases 8(1): e2286.
- Bradshaw D, Steyn K, Levitt N, Nojilana B (2011) Non-communicable diseases- A race against time. South African Medical Research Council, pp. 1-4.
- Day GE, Provost E, Lanier AP (2009) Alaska native mortality rates and trends. Public Health Reports 124(1): 54-64.
- Anderson C, Potts L (2021) Physical health conditions of the amish and intervening social mechanism: An exhaustive narrative review. Ethn Health 27(8): 1952-1978.
- Hsueh WC, Mitchell BD, Aburomia R, Pollin T, Sakul H et al. (2000) Diabetes in the old order amish: Characterization and Heritability analysis of the amish family diabetes study. Diabetes Care 23(5): 595-601.
- Hanan FAR, Abla S, Yousef K, Nahla H, Abdullatif H, et al. (2014) Non-communicable diseases in the Arab world. Lancet 383(9914): 356-367.
- Antonina T, Diana A, Stephen W, Bente M, Rakovac I, et al. (2020) Prevalence of physical inactivity and sedentary behaviour among adults in Armenia. Front Public Health 8: 157.
- World Bank Group (2013) In middle east and north Africa, health challenges are becoming similar to those in western countries.
- Yessineth DAR, Enrique AM, Juan MGT (2023) Epidemiological situation of high-prevalence non-communicable diseases in Spain: A systematic review. J Clin Med 12(22): 7109.
- Weitzman S, Lehmann E, Abu R (1974) Diabetes mellitus among the Bedouin population in the Negev. Diabetologia 10: 391.
- Jonkman LJ, Tsuchihashi K, Liu E, Lates J, Niaz Q, et al. (2020) Patient experiences in managing non-communicable diseases in Namibia. Research Social Administrative Pharmacy 16(11): 1550-1557.
- Brenda B, Paromita DR, Alejandra D, Pam L, Siobhan OD, et al. (2019) At-a-glance How healthy are Canadians? A brief update. Health Promot Chronic Dis Prev Can 39(2): 63.
- Chakraborty S (2020) Attributable burden, life expectancy and income loss to non-communicable diseases in Ireland: From evidence to policy-making. University College Cork, pp. 1-227.
- World Bank Group (2023) Noncommunicable diseases care in eastern Caribbean.
- Selvavinayagam TS, Vidhya V, Archana R, Boopathi K, Senthilkumar P, et al. (2024) Prevalence of noncommunicable disease (NCDs) risk factors in Tamil Nadu: Tamil Nadu STEPS Survey (TN STEPS), 2020. PLOS One 19(5): 0298340.
- Koupilova I, Epstein H, Holcik J, Hajioff S, Kee MM (2001) Health needs of the Roma population in the Czech and Slovak Republics. Social Science & Medicine 53(9): 1191-1204.
- Xue FH, Singh K, Kenny TA, Chan HM (2019) Prevalence of heart attack and stroke and associated risk factors among inuit in Canada: A comparison with the general Canadian population. International Journal of Hygiene and Environmental Health 222(2): 319-326.
- Cetorelli V, Burnham G, Shabila N (2017) Prevalence of non-communicable diseases and access to health care and medication among Yazidis and other minority groups displaced by ISIS into the Kurdistan region of Iraq. Conflict and Health 11: 4.
- Skeie G, Oyeyemi SO, Borch KB, Hopstock LA, Lochen ML, et al. (2022) A smartphone-based information communication technology solution for primary modifiable risk factors for noncommunicable diseases: Pilot and feasibility study in Norway. JMIR Formative Research 6(2): 33636.
- Marcia CTM, Joan S (2022) The impact of dietary changes on non-communicable diseases in Latin America. Frontiers Nutrition 9: 881676.
- Sharmi D (2024) Progress in NCD screening in Mongolia. The Lancet Oncology 25(9): 403.
- Dsouza RJ, Sunny R, Sambhalwar PB, Hariharan S, Mohanraj S, et al. (2021) Perception of noncommunicable diseases among the tribals of the Gudalur valley, Nilgiris, Tamil Nadu. Current Medical Issues 19(3): 132-136.
- Gurney J, Stanley J, Sarfati D (2020) The inequity of morbidity: Disparities in the prevalence of morbidity between ethnic groups in New Zealand. J Comorb 10: 2235042X20971168.
- Pressler M, Devinsky J, Duster M, Lee JH, Glick CS, et al. (2022) Dietary transition and health outcomes in four populations - Systematic review. Front Nutr 9: 748305.
- Bitton A, Zaslavsky AM, Ayanian JZ (2010) Health risks, chronic diseases, and access to care among US Pacific islanders. J Gen Intern Med 25(5): 435-440.
- Ruwan R, Alison W, John M, Jean PN, Pascal N, et al. (2021) Early experience in the integration of non-communicable diseases into emergency primary health care, Beni region, Democratic Republic of the Congo. Annals of Global Health 87(1): 27.
- Kraef C, Juma PA, Mucumbitsi J, Ramaiya K, Kallestrup P (2020) Fighting non-communicable diseases in East Africa: Assassing progress and identifying the next steps. BMJ Global Health 5(11): 003325.
- Niyukuri D, Nyandwi J, Kamatari O, Mikaza C, Barengayabo M (2022) A leap to non-communicable disease epidemic in Burundi: Overall trends of the disproportionate burden. MedRxiv.
- Junjie Q, Ruiqiang L, Jeroen R, Manimozhiyan A, Kristoffer SB, et al. (2010) A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464(7285): 59-65.
- Sharma A, Martins IJ (2023) The role of microbiota in the pathogenesis of alzheimer’s disease. Acta Scientific Nutritional Health 7(7): 108-118.
- Ramakrishna BS (2013) Role of the gut bacteria in microbiota in human nutrition and metabolism. J Gastroen Hepatol 28(4): 9-17.
- Dehingia M, Devi KT, Khan MR, Talukdar NC, Sharmila SM, et al. (2015) Gut bacterial diversity of the tribes of India and comparison with the worldwide data. Scientific Reports 5: 18563.
- Palmira DB, Sisto A, Lavermicocca P (2021) Probiotic bacteria and plant-based matrices: An association with improved health-promoting features. Journal of Functional Food 87: 104821.
- Alvarez MV, Bambace MF, Quintana G, Gomez ZA, María DRM (2021) Prebiotic-alginate edible coating on fresh-cut apple as a new carrier for probiotic lactobacilli and bifidobacteria. LWT 137: 110483.
- Shili Z, Yulan C, Chuzhen M, Xinyi D, Mengchen Z, et al. (2021) The role of the microbiome in diabetes mellitus. Diabetes Research and Clinical Practice 172: 108645.
© 2025 Borkar SG. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






