- Submissions

Full Text
Developments in Anaesthetics & Pain Management
Anesthetic Considerations for Advanced Neural Implants
Kunal Kumar Sharma1* and Bharti Chauhan2
1 Department of Anesthesia, Neuroanesthesia Cell, Indira Gandhi Medical College, Shimla, India
2 Department of Neuroanesthesia and Critical Care, Postgraduate Institute of Medical Education and Research, India
*Corresponding author:Kunal Kumar Sharma, Department of Anesthesia, Neuroanesthesia Cell Under, Indira Gandhi Medical college, Shimla, India
Submission:September 24, 2025;Published: October 16, 2025

ISSN: 2640-9399 Volume3 Issue1
Abstract
Spinal Cord Injury (SCI) imposes profound physical, socioeconomic and clinical burdens, with limited options for functional restoration. This manuscript explores the transformative potential of neurotechnology in addressing paralysis, spotlighting the NeuralEXO exoskeleton-a neuro-controlled, AI-integrated system designed to restore mobility by translating neural signals into mechanical motion. We examine the innovation landscape, including brain-computer interfaces, neuromodulation and regenerative therapies, supported by initiatives like the NIH BRAIN Initiative and DARPA’s N3 program. A critical focus is placed on anesthetic management during spinal implant surgeries, comparing dexmedetomidine and propofol under target-controlled infusion paradigms. Dexmedetomidine demonstrates superior preservation of motor and somatosensory evoked potentials, reduced hemodynamic instability and enhanced recovery profiles, though propofol remains valuable for its titratability. Challenges such as autonomic dysreflexia, signal latency and device safety are addressed, emphasizing the need for interdisciplinary collaboration and robust clinical protocols. By integrating advanced neurotechnology with optimized intraoperative care, this work underscores a path toward redefining functional recovery in SCI.
Keywords:Spinal cord injuries; Exoskeleton device; Brain-computer interfaces; Neuromodulation
Introduction
Spinal Cord Injury (SCI) remains one of the most devastating neurological conditions, frequently resulting in permanent paralysis, sensory loss and profound impairment of motor function. Traumatic disruption of descending neural pathways interrupts brain-derived motor commands, culminating in lifelong disability. Despite advances in surgery, intensive care and rehabilitation, functional restoration remains limited. Globally, traumatic SCI occurs at an estimated incidence of 11.5 cases per 100,000 persons per year, with lifetime costs ranging from € 91,000 to € 455,000 per patient depending on injury severity [1]. This socioeconomic and clinical burden underscores the urgent need for innovative strategies capable of restoring mobility and independence. Paralysis itself represents a “Blue Ocean” in healthcare innovation. The unmet clinical need is striking: few effective treatments exist to reverse paralysis, yet demand for solutions is immense among patients, families and health systems. In the United States alone, approximately 5.4 million individuals-about one in every fifty-live with some form of paralysis. Motor vehicle collisions account for 38% of new cases annually, followed by falls (30%), violence (13%), sports (9%) and medical or surgical complications (5%) [2]. Beyond physical limitations, paralysis carries deep socioeconomic consequences: 28.1% of households with affected members report annual incomes below $15,000, significantly higher than the general population [3]. Current management remains largely supportive, with modest competition in transformative interventions. Only a handful of companies-such as Medtronic, Natus and Neuralink-actively innovate in neurostimulation, neuroprosthetics or consciousness mapping [4].
Parallel efforts in anesthetic management and neuroprotective strategies aim to mitigate secondary injury, reduce neuroinflammation and promote neuroplasticity, though these remain early in translation. Simultaneously, societal focus on brain health and government-backed programs, such as the U.S. National Institutes of Health BRAIN Initiative, the Defense Advanced Research Projects Agency (DARPA) and the Next-Generation Nonsurgical Neurotechnology (N3) program, have accelerated the innovation pipeline [5,6]. Within this evolving landscape, advanced exoskeletons, brain-computer interfaces, neuromodulation platforms and regenerative therapies converge on the shared goal of restoring autonomy in paralysis. We highlight the intersection of cutting-edge neurotechnology, exemplified by the NeuralEXO exoskeleton project, with the anesthetic and intraoperative considerations required for safe implantation of spinal implants [7]. In particular, we focus on dexmedetomidine and propofol under Target-Controlled Infusion (TCI) paradigms, synthesizing evidence for their impact on evoked potential monitoring and clinical outcomes. Additionally, we project future directions, including closed-loop brain-spinal interfaces and non-invasive neuromodulation, underscoring how anesthetic management remains a cornerstone of innovation in neurorehabilitation.
Innovation Landscape
The past decade has witnessed rapid growth in Artificial Intelligence (AI), neuromonitoring and digital therapeutics in neurocritical care. AI platforms increasingly support prediction of coma recovery, optimization of sedation and analysis of evoked potentials or Electro Encephalo Graphy (EEG). Intraoperative neuromonitoring is now integral to neurosurgery, enabling early detection of subarachnoid hemorrhage, vasospasm or infarction-sometimes preceding imaging. Predictive models for neuromonitoring [8,9] have proliferated in recent years, underscoring the clinical relevance of computational neuroscience. Brain-Computer Interfaces (BCIs) represent another transformative frontier, restoring motor functions and enabling communication in locked-in patients, including those with amyotrophic lateral sclerosis [10,11]. Concurrently, wearable neurotechnologies now permit home-based monitoring and neurofeedback for patients with disorders of consciousness [12]. However, most algorithms remain validated only in younger to middle-aged populations (14- 60 years), raising concerns regarding generalizability to elderly cohorts where SCI is increasingly prevalent. Virtual Reality (VR) and Augmented Reality (AR) systems are emerging as critical tools for post-paralysis recovery, particularly in immersive rehabilitation environments. Predictive analytics platforms further extend value in intensive care units by enabling outcome stratification for comatose patients. On the regenerative horizon, stem cell therapy, gene editing and pharmacological neuroplasticity enhancers continue to advance, aiming to restore or augment neural circuitry after injury [13]. Together, these domains constitute an integrated innovation ecosystem spanning devices, software and biologics.
Human-Centric Exoskeleton Innovation
NeuralEXO is envisioned as an advanced neuro-controlled exoskeleton aimed at restoring mobility and autonomy for individuals with paralysis. Unlike passive orthotic aids, it integrates Electro Encephalo Graphy (EEG) and Electro Myo Graphy (EMG) signals for real-time control, translating neural intent into mechanical motion. Iterative design leverages servo motors and actuators in modular frameworks, ensuring adaptability across diverse patient anatomies. Additive manufacturing and precision robotics support scalable production, while AI-driven signal decoding underpins exoskeletal actuation. Clinical integration will prioritize rehabilitation centers treating SCI, stroke, and neuromuscular disorders. Beyond healthcare, defense and veteran rehabilitation programs represent secondary markets, where restoring mobility aligns with functional reintegration goals. Core challenges include minimizing signal processing latency, adapting to patient-specific neurophysiology and reducing device weight while preserving structural integrity. Safety-critical features, such as emergency stop functions, actuator redundancies and predictive diagnostics, are mandatory for clinical trust. Compliance with ISO 13485 and IEC 60601 standards, alongside FDA 510(k) clearance, will ensure regulatory and patient safety benchmarks. Commercial strategies balance direct sales to hospitals with leasing models for clinics and home use. Service contracts covering software updates, recalibration and maintenance provide secondary revenue streams. Interdisciplinary teams are central to NeuralEXO’s success, comprising neuroengineers, neuroanesthetists, neurologists, physiotherapists and roboticists. Human factors engineering, intuitive interface design, and clinician training programs will be essential for adoption. Performance indicators include prototype iteration cycles, successful clinical trial sessions and user training completion rates. Ultimately, NeuralEXO is designed to evolve through modular upgrades and continuous clinician-patient feedback.
Anesthetic Considerations
While implant design and surgical precision are critical, anesthetic management during implantation plays an equally pivotal role. High-quality intraoperative neuromonitoring-particularly Motor Evoked Potentials (MEPs) and Somatosensory Evoked Potentials (SSEPs)-requires agents that preserve signal fidelity. Simultaneously, patients with SCI are vulnerable to hemodynamic instability due to autonomic dysfunction, making anesthetic choice a determinant of both safety and implant efficacy. Dexmedetomidine, a selective α₂-adrenergic agonist, provides sedation, anxiolysis and analgesia with minimal respiratory depression. Its modest, dosedependent effects on evoked potentials make it attractive for spinal surgery. The Hannivoort TCI model has demonstrated feasibility for plasma-site targeting, supporting integration into TCI paradigms. Propofol, a GABAA receptor agonist, remains a mainstay due to rapid onset and offset, enabling tight titration. However, its dosedependently suppresses MEP amplitudes, particularly at higher infusion rates, often necessitating “propofol-sparing” strategies. Hemodynamic instability-especially hypotension and respiratory depression-poses additional concerns in SCI. The Eleveld TCI model provides improved pharmacokinetic prediction across ages compared with Schnider models, with effect-site concentrations of 5-8μg/ml commonly used in spine surgery [14]. While sensory EPs may be preserved at moderate doses, motor EPs are more vulnerable. Recovery is typically rapid but opioid requirements are higher due to lack of intrinsic analgesia. Terao et al. [15] compared dexmedetomidine and propofol in patients undergoing various spinal surgeries.
The analysis revealed that dexmedetomidine provided superior sedation, less interference with evoked potentials and a reduced incidence of hypotension compared to propofol. Patients receiving dexmedetomidine also had a faster recovery time and lower postoperative pain scores [15]. Ter Bruggen et al. [16] found that dexmedetomidine, compared to propofol, was associated with improved intraoperative neuromonitoring outcomes, including better preservation of EP signals during spinal cord stimulator placement [16]. Mahamoud et al. [17] noted that propofol compromised EP clarity, while dexmedetomidine maintained stable recordings [17]. However, pediatric evidence remains mixed. Holt et al. [18] showed that dexmedetomidine at 0.3-0.5μg/kg/hr reduced MEP amplitudes during pediatric spine fusion, though SSEPs were unaffected [18]. Tobias et al. [19] using a combined propofolremifentanil- dexmedetomidine protocol, demonstrated preserved neurophysiological monitoring when propofol dosing was adjusted [19]. A retrospective study by Beňuška et al. [20] highlighted the importance of integrating latency delays with amplitude reductions as alarm criteria during MEP monitoring. Such refinements illustrate how anesthetic protocols, signal thresholds and monitoring criteria converge to determine intraoperative safety [20]. Nevertheless, Sharma et al. [21] did not observe any significant alteration in the MEPs when using dexmedetomedine as the prime anesthetic induction and maintenance agent in their case series [21,22]. Liu et al. [23] found that propofol provides adequate sedation and amnesia and minimally affects amplitude and latency of sensory evoked potentials during the procedure [23]. However, Kim et al. [24] found that propofol infusion led to greater hemodynamic instability compared to other agents, such as dexmedetomidine, during surgeries that require constant neurological monitoring [24].
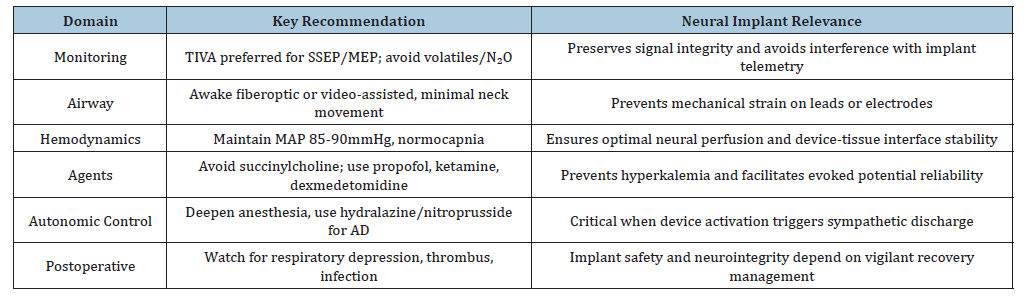
Propofol does not have the analgesic properties of dexmedetomidine and thus, postoperative pain management often requires additional analgesics. Wong et al. [25] observed that patients who received propofol for anesthesia required higher doses of postoperative opioids, which may lead to increased risk of opioid-related side effects and delayed recovery [25]. The implication from the work by Dooney et al. [26] emphasizes the need for continuous multimodal neurophysiological monitoring for patients with Deep Brain Stimulators (DBS) (Table 1), spinal cord stimulators or brain-computer interface electrodes, where anesthetic choice must preserve electrophysiologic signal fidelity and implant function. Mekkat et al. [27] have elaborated the clinical challenges associated with managing autonomic dysreflexia in patients with spinal cord injury. Autonomic dysreflexia also acts as a challenge with implant activation and brain stimulation during surgery. Thereby emphasizing vigilance to prevent dysreflexia or hypertensive crises during intraoperative testing. From the works by Bao & colleagues [28] on spinal cord injury patients, we can likewise emphasize the preference of TIVA for patients undergoing epidural stimulator implants and DBS placement. This primarily due to the issue that volatile anesthetic agents and N₂O depress synaptic transmission thereby obscuring device calibration and evoked potentials. For cervical cord stimulators, neck stabilization, controlled induction and avoidance of fasciculations are essential to prevent lead displacement and neural interface microtrauma. The patients implanted with closed-loop spinal or cortical devices require stable perfusion to prevent ischemic signal distortion or electrode interface injury. The need for early mobilization and thromboprophylaxis while maintaining implant site sterility to prevent infection forms a critical aspect in these patients. Table 2 summarizes the implant related anesthetic considerations.
Table 1:Comparative summary between dexmedetomidine and propofol.
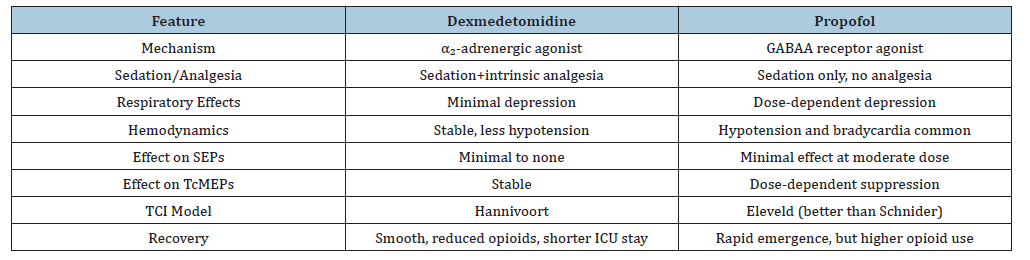
Table 2:Summary table for neural implant–related anesthesia principles.
Semantics
Emerging evidence suggests dexmedetomidine provides more stable hemodynamics and smoother recovery compared with propofol, making it attractive for advanced spinal implant surgeries. Nonetheless, propofol’s titratability and established familiarity ensure its continued relevance. Direct randomized comparisons under TCI paradigms remain essential to establish evidence-based guidelines.
Conclusion
The landscape of SCI rehabilitation is undergoing rapid transformation, driven by advances in neurotechnology, digital therapeutics and regenerative biology. NeuralEXO exemplifies the promise of human-centric, AI-integrated exoskeletal systems designed to restore mobility and independence. Yet the success of such devices depends not only on engineering precision and clinical design, but equally on anesthetic management during implantation. Incorporating conventional neuromonitoring techniques like regional oximetry for spine implants shall enhance their success further [29,30]. In light of emerging evidence in favor of dexmedetomidine, systematic randomized studies are required to formalize best practice for such procedures. The bridging of surgical precision, anesthetic safety and technological innovation shall ensure that the next generation of implants hold the potential to redefine functional recovery in spinal cord injury.
References
- Liu Y, Yang X, He Z, Li J, Li Y, et al. (2023) Spinal cord injury: Global burden from 1990 to 2019 and projections up to 2030 using bayesian age-period-cohort analysis. Front Neurol 14: 1304153.
- Spinal Cord Injury (SCI) 2016 facts and figures at a glance. J Spinal Cord Med 39(4): 493-494.
- Armour BS, Courtney Long EA, Fox MH, Fredine H, Cahill A (2016) Prevalence and causes of paralysis-united states, 2013. Am J Public Health 106(10): 1855-1857.
- Varma AK, Das A, Wallace G, Barry J, Vertegel AA, et al. (2013) Spinal cord injury: A review of current therapy, future treatments and basic science frontiers. Neurochem Res 38(5): 895-905.
- Bargmann CI, Newsome WT (2014) The brain research through advancing innovative neurotechnologies (brain) initiative and neurology. JAMA Neurol 71(6): 675-676.
- Miranda RA, Casebeer WD, Hein AM, Judy JW, Krotkov EP, et al. (2015) DARPA-funded efforts in the development of novel brain-computer interface technologies. J Neurosci Methods 244: 52-67.
- He Y, Xu Y, Hai M, Feng Y, Liu P, et al. (2024) Exoskeleton-assisted rehabilitation and neuroplasticity in spinal cord injury. World Neurosurg 185: 45-54.
- Sharma KK, Reddy KRM (2025) Exploratory modeling of intraoperative co-oximetry data for predicting hemodynamic trends in a thalassemic patient: A pilot case. Journal of Neuroanaesthesiology and Critical Care 12(4): 1-5.
- Kumar K, Gopalakrishna KN (2026) Predictive modeling of intraoperative SSEP during ACOM aneurysm clipping: A case-based simulation stud. Abstracts from the 53rd annual meeting of the society for neuroscience in anesthesiology and critical care. J Neurosurg Anesthesiol 38(1): e1-e57.
- Metzer SL, Littlejohn KT, Silva AB, Moses DA, Seaton MP, et al. (2023) A high-performance neuroprosthesis for speech decoding and avatar control. Nature 620(7676): 1037-1046.
- Bajaj R, Sharma KK (2024) Neuralink and neuro-monitoring concerns in neuroanaesthesia. Ind J Anesth Analg 11(4): 230-232.
- Autenrieth M, Kober SE, Wood G (2023) Assessment of the capacity to modulate brain signals in a home-based SMR neurofeedback training setting. Frontiers in Human Neuroscience 16: 1032222.
- Dwivedi S, Choudhary P, Gupta A, Singh S (2023) Therapeutical growth in oligodendroglial fate induction via transdifferentiation of stem cells for neuroregenerative therapy. Biochimie 211: 35-56.
- Eleveld DJ, Colin P, Absalom AR, Struys MMRF (2018) Pharmacokinetic-pharmacodynamic model for propofol for broad application in anesthesia and sedation. Br J Anaesth 120(5): 942-959.
- Terao Y, Ichinomiya T, Higashijima U, Tanise T, Miura K, et al. (2012) Comparison between propofol and dexmedetomidine in postoperative sedation after extensive cervical spine surgery. J Anesth 26(2): 179-186.
- Ter Bruggen FFJA, Ceuppens C, Leliveld L, Stronks DL, Huygen FJPM (2019) Dexmedetomidine vs propofol as sedation for implantation of neurostimulators: A single-center single-blinded randomized controlled trial. Acta Anaesthesiol Scand 63(10): 1321-1329.
- Mahmoud M, Sadhasivam S, Salisbury S, Nick TG, Schnell B, et al. (2010) Susceptibility of transcranial electric motor-evoked potentials to varying targeted blood levels of dexmedetomidine during spine surgery. Anesthesiology 112(6): 1364-1373.
- Holt F, Strantzas S, Zaarour C, Chamlati R, Vreugdenhil I, et al. (2020) The effect of dexmedetomidine on motor-evoked potentials during pediatric posterior spinal fusion surgery: A retrospective case-control study. Canadian Journal of Anesthesia 67(10): 1341-1348.
- Tobias JD, Goble TJ, Bates G, Anderson JT, Hoernschemeyer DG (2008) Effects of dexmedetomidine on intraoperative motor and somatosensory evoked potential monitoring during spinal surgery in adolescents. Paediatric anaesthesia 18(11): 1082-1088.
- Beňuška J, Čembová N, Naser Y, Žabka M, Pasiar J, et al. (2020) Evaluation of a combination of waveform amplitude latency and decrease of waveform amplitude magnitude during spinal surgery in intraoperative neurophysiological monitoring of Transcranial motor evoked potentials and its incidence on postoperative neurological deficit. Acta Chirurgiae Orthopaedicae Et Traumatologiae Cechoslovaca 87(1): 39-47.
- Sharma KK, Sharma S, Takkar V, Devi M, Krishnaswami S (2025) Neurocognition after electroencephalography guided anesthetic induction with dexmeditomidine in neurosurgical patients: A case series. J Neuroscience and Neurological Surgery 17(3): 1-6.
- Sharma KK, Guleria S, Dhatwalia P, Singh B, Thakur DS, et al. (2025) Total intravenous anesthesia versus volatile anesthetic maintenance for ACDF surgeries-an observational study. J Sur Anesth Res 6(8): 1-4.
- Liu EH, Wong HK, Chia CP, Lim HJ, Chen ZY, et al. (2005) Effects of isoflurane and propofol on cortical somatosensory evoked potentials during comparable depth of anaesthesia as guided by bispectral index. Br J Anaesth 94(2): 193-197.
- Kim MH, Park J, Park YG, Cho YE, Kim D, et al. (2025) Comparison of intraoperative neurophysiological monitoring between propofol and remimazolam during total intravenous anesthesia in the cervical spine surgery: A prospective, double-blind, randomized controlled trial. Korean J Anesthesiol 78(1): 16-29.
- Wong SSC, Choi EKY, Chan WS, Cheung CW (2022) Propofol total intravenous anaesthesia versus inhalational anaesthesia for acute postoperative pain in patients with morphine patient-controlled analgesia: A large-scale retrospective study with covariate adjustment. BMC Anesthesiol 22(1): 140.
- Dooney N, Dagal A (2011) Anesthetic considerations in acute spinal cord trauma. Int J Crit Illn Inj Sci 1(1): 36-43.
- Mekkat A, Kristo G (2024) Anesthesia considerations in spinal cord injury patients: An overview. Glob J Surg Case Rep.
- Bao FP, Zhang HG, Zhu SM (2017) Anesthetic considerations for patients with acute cervical spinal cord injury. Neural Regen Res 12(3): 499-504.
- Sharma KK, Sharma P, Surve RM (2024) Cerebral oxygenation monitoring for early detection of subarachnoid haemorrhage in infratentorial arteriovenous malformation undergoing embolisation: A case study. Indian J Anaesth 68(2): 206-208.
- Amiri AR, Lee CH, Leung TS, Hetreed M, Craggs MD, et al. (2013) Intraoperative assessment of human spinal cord perfusion using near infrared spectroscopy with indocyanine green tracer technique. Spine J 13(12): 1818-1825.
© 2025 Kunal Kumar Sharma. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






