- Submissions

Full Text
Clinical Research in Animal Science
Review on Epidemiological Distribution of Economically Important Nematodes in Ethiopia, with Particular Emphasis to Haemonchus Species
Kassahun Berhanu*
Department of Parasitology and Pathology, Ethiopia
*Corresponding author: Kassahun Berhanu, Department of Parasitology and Pathology, College of Veterinary Medicine, Jigjiga, Ethiopia
Submission: July 07, 2022;Published: October 03, 2022

ISSN: 2770-6729Volume 2 - Issue 3
Abstract
Suitable climatic condition found in tropical areas create wide spread of gastro-intestinal nematode of ruminants. The epidemiology of gastro-intestinal nematodes mainly depends on the climatic condition (temperature and humidity) of environment. Ethiopia is among the country with high occurrence of gastro-intestinal nematode infection in the region. According to different studies, the prevalence of nematodes in cattle ranges from 27-51% and 21.35%-96.7% in sheep and goat. The prevalence of Haemenchus contortus in sheep and goat ranges between 40% and 91.2%. Different studies indicated that; Agro-ecology, season, climate, host factor and management systems are the main factor for variation in prevalence across the country. The high prevalence of gastro-intestinal infection in the country have direct effect on livestock production causing mortality, decreasing growth rate and milk production, increasing treatment cost. Due to that effective control and preventive strategies should be required to tackle the devastating effect of the gastro-intestinal nematodes on livestock production.
Introduction
Livestock production plays important role in both national and livelihood economies of Ethiopia. The country has owned huge livestock population estimates that 65.35 million heads of cattle, 39.89 million sheep, and 50.50 million goats [1]. This huge livestock population cannot be properly utilized due to clinical and non-clinical parasitic diseases which causing mortality, weight loss, decreasing in growth and productivity [2]. The distribution and prevalence of the parasitic disease should be presented by geographical areas that could roughly correspond to climatic conditions. For instance, presence of suitable ecological condition (temperature, humidity, vegetation, rain fall) in Ethiopia, brought high prevalence of GIT nematodes [3]. Different studies shows that climate condition play important role in ecology of GIT nematodes by facilitating transmission and survival of eggs and larvae of parasites [4]. The well organized and comprehensive data on the epidemiology of GIT nematodes in Ethiopia is very essential for researchers and policy makers to develop strategies on control and prevention systems in livestock. So, this review aimed to bring available data together and indicate the epidemiology and prevalence of major GIT nematodes in ruminants in Ethiopia. About 40 articles from main electronic data bases (Google scholar and PubMed) were used to develop this review
Gastro-Intestinal Parasites (Nematodes) of Ruminants
Nematodes (round worms) are free-living unsegmented worms, which have cylindrical form, tapering at either ends. Their body is covered with a colorless, somewhat translucent layer called the cuticle, are elongated in shape and an alimentary canal is present. They have separate sexes and exhibit both direct and indirect life cycle, are found in fresh water, the sea and the soil and are among the most successful parasites of plants and animals. Gastrointestinal nematodes of greatest importance in ruminants are Haemonchus, Ostertagia, Trichostrongylus, Cooperia, Nematodirus, Bunostomum (hookworms), Strongyloides, Oesophagostomum, Chabertia ovina, Trichuris, (whipworms) Dictyocaulus, Parafilaria, Onchocerca, Protostrongylus, Muellerius, Ascaris, Thelazia and others [5].
Life cycle
Most Trichostrongyle group of nematodes (Trichostrongyles, Oesophagostomum and Bunostomum) have direct life cycle (complete their life cycle in one host). The eggs produced by female parasites passed out with faeces and emberyonation takes place in environment in optimal conditions. The embrynated eggs hatched to 11, 12 and 13 (infective stage) in 7 to 10 days under suitable temperature and humidity. Ruminants are infected during grazing by ingesting L3 (infective stage) and the larvae passed to abomasum or intestines. Then, the extra cuticle is removed [5] and penetrate to different organs such as mucous membrane (Trichosrongylus and hemonchus), gastric glands (ostertagia), lamina propria (Oesophagostomum) However, the infection in Trichuris caused after ingesting larvated egg by host and the larva is released in the intestines. The time period from the ingestion of infective larvae to the egg production by adult female parasite is called prepatent period. Different species of parasite have require different time period. For examples Haemonchus placei (cattle) for 3-4 weeks, Haemonchus contortus (sheep), two weeks, Ostertagia (sheep and cattle) for 3 weeks. GIT nematodes have different egg producing capacities. The number of eggs released with faeces depending on species of parasites, host immunity, level of infection and number of adult parasites found in GIT system [6]. Larvae released from female parasites require optimum temperature (10-36°C) and humidity (85%) for development and survival in the environment.
In tropical and sub-tropical climate, temperature have play major role. During rainy seasons; the free living infective stages (eggs, larvae, cysts, and oocysts) of survival, hatching rate and development are increased. However, adverse climatic condition in dry season decrease the prevalence of parasite infections because the larvae arrested in the host and environment [7]. The development of larvae to infective stage take place in fecal material. The infective larvae migrated or transported horizontally or vertically to the nearby herbage to be ingested by hosts [8].
Prevalence of Nematodes in different parts of Ethiopia
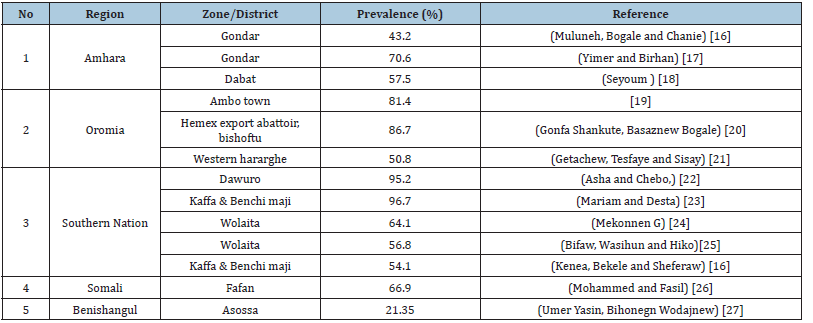
Many cross sectionals study on nematodes of cattle were carried out in many part of Ethiopia. [9-15]. According to (Table 1), the study in different parts of Ethiopia indicated that the overall prevalence parasitic infection of cattle ranges from 27- 51%. According to different studies in Ethiopia, the prevalence of GIT nematode in small ruminants ranges from 21.35% to 96.7% depending on production systems (Table 2). The higher prevalence of helminth parasites infection of cattle, sheep and goat were reported from different parts of Ethiopia [16-27]. According to the above studies, the distribution of the parasites across Ethiopia varies depending on agroecology, seasonal variation, climate, management systems and host factors like age, body condition and breed of animals. For instance, the prevalence of the parasites egg count is higher in wet season than dry season. This confirm the fact that the parasite require the conducive environment for the survival, development and transmission. Most studies agrees on high prevalence of nematodes in animal with poor body condition than good body condition due to low immunity level. Higher prevalence also reported in sheep than goat due to grazing behavior of sheep that expose them to infective stage of larvae.
Table 1:Prevalence of GIT nematodes of cattle in Ethiopia.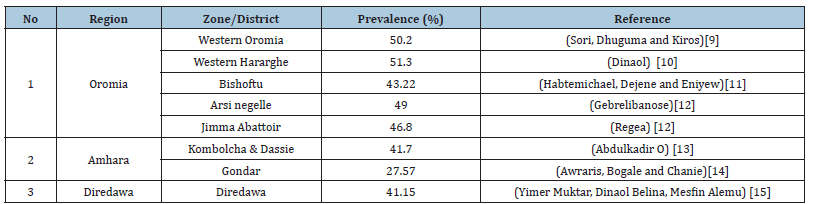
Table 2:Spatial distribution of nematodes of small ruminants in Ethiopia.
Haemonchosis of Large and Small Ruminants in Ethiopia
The genus Haemonchus grouped under sub-family Haemonchinae. Domestic rumininants are mainly infected by four main species namely, Haemonchus contortus (sheep and goat), Haemonchus placei and Similis (cattle) and Haemonchus longistepis (Camelus dromederi). Adult parasite of Haemonchus contortus found in the abomasum and commonly known as barber pole or wire worm [8]. The parasite have direct life cycle. High fecundity of female worm have determine the epidemiology of Haemonchosis in addition to warm and humid condition. In favorable conditions (wet season), the infective larvae increased in the pasture. In Ethiopia, high prevalence was recorded in and around two rainy season (May and September) of the year. Because the condition favorable for the transmission, development and survival of free-living stage of parasites [28-33]. In addition, the microclimate of feces and herbage are also important for larval development and survival [34]. Haemonchus contortus cause Haemonchosis in sheep and goat. The disease is highly prevalent, pathogenic and economically important. According to Arsenopoulos et al. [3] the annual cost for treatement of the disease in sheep and goat estimated to 103 million USD in India, 46 million USD in the Republic of South Africa and 26 million USD in Kenya.
Prevalence and spatial distribution of hemonchosis in sheep and goat across Ethiopia
Different studies revealed that the prevalence of Hemonchus Contortus in Ethiopia ranges from 40%-91.2% (Table 3). There is variation in prevalence across different parts of Ethiopia. Different agro-climatic conditions, management system and sample size among the factors for variation in prevalence. And also, grazing type, stocking density and nutritional status might be a reason for difference in prevalence [6]. The higher prevalence of Haemonchosis from different parts of Ethiopia indicate the high distribution of the parasite through the country (Table 3). From different studies, higher prevalence of parasite reported in sheep than goat [35]. As they justify their reason; ground grazing habit of sheep helps acquisition of more infective larvae (L3) and the fact that goats browse on bushes and small trees decrease the acquisition to infective larvae.
Table 3:Prevalence of Hemonchus species in different parts of Ethiopia.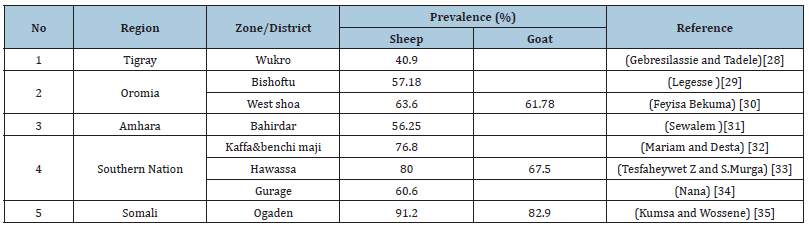
Factors influencing the epidemiological distribution of haemonchus infection in small ruminants
Pathogen factors: Haemonchus contortus have various strategies to overcome the adverse effect come from the environment and host. The female haemonchus produce large number of eggs than other female parasite. Though, this result over contamination of grazing areas and brought animal rapid reinfection [36]. The parasite have also unique feature (Hypobiosis) during unfavorable condition to minimize the mortality of free living stages. Photo-period used as induction signal for hypobiosis after infection by L3. This aspect of gastrointestinal nematodes epidemiology known till now only for abomasal nematodes needs further validation for other gastrointestinal nematodes parasites and in other host species so as to arrive at a certain decisive conclusion about the role of hypobiosis (arrested development) in the onset of diseases, their epidemiology and their effective control and treatment [37]. Basically, climatic conditions may result in two distinct patterns of inhibition: ‘winter inhibition’ in which nematodes inhibit development before the winter in temperate regions and ‘summer inhibition’ in which the onset of inhibition is before summer or before a dry season. This pattern of development enables the abomasal nematodes to live longer in the host avoiding the harsh climatic conditions and presents an interesting example of an ecological adaptation of a para- site to its local climatic conditions [38].
Host factor:The severity of infection determined by different Intrinsic factor (Age of animals, breed, sex, genetics and physiological status of host. Pasture contamination aggravate the severity for grazing animals. Due to low immunity, new born animals become more susceptible to infection. However, immunological maturity and repeated exposure lower the occurrence in adult animals. In Ethiopia, some local breed of sheep (Menz, Horro, Ferta and Afar) and goat (Kefa, Abergeile and Begait) are relatively resistant to GIT nematodes than exotic breeds [39]. In addition to host factor, the suitable environmental condition (optimum temperature and humidity) have important contribution for larval development in tropical countries like Ethiopia [40].
Control and Prevention Strategies
The knowledge about the epidemiology of GIT nematodes infection in ruminants is crucial for controlling strategies. Because the development and survival of infective larvae determined by climatic condition (temperature and humidity). The control method against the parasite taken place on host and environment require seasonal considerations. Due to that the timing and frequency of anthelmintic treatments depends on the level of parasitism and epidemiology of parasitic species in a given climatic condition. (Hansen, 1994). Generally, pasture management, provision of proper feed and clean water, regular deworming have minimize losses caused by GIT nematodes control the parasitic infection [41].
- (2020) Central Statistical Authority of Ethiopia: Report on Livestock and Livestock Characteristics (Private Peasant Holdings).
- Gebrelibanose BH (2016) Study on Distribution of Gastrointestinal Nematodes and Coccidian Parasites of Cattle in West Arsi zone, Ormia Regional State, Ethiopia. Journal of V Journal of Veterinary Science & Journal of veterinary Science & Technology 5(5): 1-6.
- Bifaw A, Wasihun P, Hiko A (2018) Small ruminants gastrointestinal nematodiasis with species composition identification in Humbo District, Wolaita Zone, Ethiopia. Journal of Veterinary Science & Technology 9(6): 1-7.
- Pfukenyi DM, Mukaratirwa S (2013) A review of the epidemiology and control of gastrointestinal nematode infections in cattle in Zimbabwe. Onderstepoort journal of veterinary research 80(1): 1-12.
- Meyer KF (1956) Veterinary parasitology. The American Journal of Tropical Medicine and Hygiene 5(5): 934-934.
- Abebe T, Yobsan T, Debela A (2018) Prevalence of major gastrointestinal nematode and degree of parasite infestation in sheep of Bako agricultural research center community-based breeding program project small holder farms at Horro district. Dairy Vet Sci J 8(3): 555740.
- Woldeyes B (2021) Review on major gastrointestinal nematodes of small ruminants in Ethiopia. International Journal of Advanced Research in Biological Sciences 8(2): 58-71.
- Hansen J, Perry BD (1994) The epidemiology, diagnosis and control of helminth parasites of ruminants. The International Laboratory for Research on Animal Diseases: 1-129.
- Regassa F, Sori T, Dhuguma R, Kiros Y (2006) Epidemiology of gastrointestinal parasites of ruminants in Western Oromia, Ethiopia. International journal of applied Research in Veterinary Medicine 4(1): 51-57.
- Belina D, Giri A, Mengistu S, Eshetu A (2017) Gastrointestinal nematodes in ruminants: The parasite burden, associated risk factors and anthelmintic utilization practices in selected districts of east and western Hararghe, Ethiopia. Journal of Veterinary Science and Technology 8(2): 433-439.
- Yonas GH, Meron D, Solomon ME (2018) Prevalence of gastrointestinal helminth parasites and identification of major nematodes of cattle in and around Bishoftu, Oromia Region, Ethiopia. Journal of Veterinary Medicine and Animal Health 10(7): 165-172.
- Regea G (2019) Prevalence of major gastrointestinal tract parasite of cattle at municipal abattoir of JIMMA town, Oromia, Southwestern Ethiopia. Vet Med Open J 4(1): 36-44.
- Abdulkadir O, Hamid M, Alemayehu A, Tintagu T (2017) Journal of Veterinary Science & Technology.
- Awraris T, Bogale B, Chanie M (2012) Occurrence of gastrointestinal nematodes of cattle in and around Gondar town, Amhara regional state, Ethiopia. Acta Parasitologica Globalis 3(2): 28-33.
- Muktar Y, Belina D, Alemu M, Shiferaw S, Belay H (2015) Prevalence of gastrointestinal nematode of cattle in selected Kebeles of Dire Dawa districts eastern Ethiopia. Advances in Biological Research 9(6): 418-423.
- Muluneh J, Bogale B, Chanie M (2014) Major gastrointestinal nematodes of small ruminants in Dembia District, Northwest Ethiopia. European Journal of Applied Sciences 6(2): 30-36.
- Yimer A, Birhan E (2016) Prevalence and identification of gastrointestinal nematodes of small ruminants in northern Ethiopia. Middle-East Journal of Scientific Research 24(8): 2602-2608.
- Seyoum Z, Getnet K, Chanie M, Derso S, Fentahun S (2018) Morbidity parameters associated with gastrointestinal tract nematodes in sheep in Dabat district, Northwest Ethiopia. Biomed Research International 2018: 9247439.
- Moges S, Hebtom K, Gashaw B, Melkamu T, Sefefe T (2017) Prevalence of Haemonchus contortus of Sheep Slaughtered at Bahir Dar Municipal Abattoir, Bahir City, Ethiopia. Global Veterinaria 18(4): 269-276.
- Temesgen A, Walanso I (2015) Major gastrointestinal helminth parasites of grazing small ruminants in and around Ambo town of Central Oromia, Ethiopia. Journal of Veterinary Medicine and Animal Health 7(2): 64-70.
- Getachew M, Tesfaye R, Sisay E (2017) Prevalence and risk factors of gastrointestinal nematodes infections in wukro. Journal of Veterinary Science & Technology 8(2).
- Asha A, Chebo B (2015) Epidemiological study on gastrointestinal tract helminthosis of small ruminants in Dawuro zone. Ethiopian Veterinary Journal 19(1): 63-82.
- Desta HMG (2015) Study on prevalence of GI nematodes in indigenous bonga sheep breed at three selected agro ecologies of Kaffa and Bench Maji Zones, Ethiopia. Journal of Biology, Agriculture and Healthcare 5(13): 77-85.
- Mekonnen G (2020) Prevalence of gastrointestinal nematodes in small ruminants in Boloso Sore District, Wolaita Zone, Southern Ethiopia. Journal of Veterinary Medicine and Animal Sciences 3(1): 1034.
- Bifaw A, Wasihun P, Hiko A (2018) Small ruminants gastrointestinal nematodiasis with species composition identification in Humbo District, Wolaita Zone, Ethiopia. Journal of Veterinary Science & Technology 9(6): 1-7.
- Kenea T, Bekele J, Sheferaw D (2015) Gastro-intestinal nematodes of sheep and goats in three districts of Kaffa and Bench Maji Zones, Southwest Ethiopia. Ethiopian Veterinary Journal 19(2): 67-76.
- Mohammed A, Fasil N (2019) The prevalence of ovine gastrointestinal nematode and associated risk factor in jigjiga Woreda, Fafan Zone, Somali Regional State, Eastern Ethiopia. Journal of Dairy & Veterinary Sciences 11(1): 555802.
- Yasin U, Wodajnew B, Tsehaineh D (2017) Study on the prevalence of GIT nematode infection of small ruminants in Kurmuk Woreda, Assosa Zone of Benishangul Gumuz Region, Western Ethiopia. Rep Opin 9(10): 48-59.
- Selemon M (2018) Review on control of Haemonchus contortus in sheep and goat. Journal of Veterinary Medicine and Research 5(5): 1139.
- Woldeyes B (2021) Review on major gastrointestinal nematodes of small ruminants in Ethiopia. International Journal of Advanced Research in Biological Sciences 8(2): 58-71.
- Gebresilassie L, Afera TB (2015) Prevalence of ovine haemonchosis in Wukro, Ethiopia. Journal of parasitology research 2015: 635703.
- Workineh L, Takele B, Fanta D, Debela T, Yacob HT (2019) Prevalence of Haemonchus contortus in sheep and goats slaughtered at Modjo Luna export Abattoir, Bishoftu, Ethiopia. International Journal of Advanced Research in Biological Sciences 6(9): 13-19.
- Bekuma F, Dufera B (2019) Prevalence of heamonchosis in small ruminants and its associated risk factors in and around Ejer e Town, West Shoa, Oromia, Ethiopia. American Journal of Biomedical Science & Research 3(5): 409-414.
- Belina D, Giri A, Mengistu S, Eshetu A (2017) Gastrointestinal nematodes in ruminants: The parasite burden, associated risk factors and anthelmintic utilization practices in selected districts of east and western Hararghe, Ethiopia. Journal of Veterinary Science and Technology 8(2): 433-439.
- Desta HMG (2015) Study on prevalence of GI nematodes in indigenous bonga sheep breed at three selected agro ecologies of Kaffa and Bench Maji Zones, Ethiopia. Journal of Biology, Agriculture and Healthcare 5(13): 77-85.
- Tesfaheywet Z, Murga S (2019) Prevalence, species composition and worm burden of abomasal nematodes of small ruminants slaughtered in Hawassa, Southern Ethiopia. African Journal of Food, Agriculture, Nutrition & Development 19(4).
- Nana T (2016) Prevalence of ovine gastrointestinal nematodes in Meskan district, Gurage zone, Southern Ethioipa. Journal of Natural Sciences Research 6(15): 1-8.
- Kumsa B, Wossene A (2007) Abomasal nematodes of small ruminants of Ogaden region eastern Ethiopia: Prevalence, worm burden and species composition. Revue de Médecine Vétérinaire 158(1): 27.
- Arsenopoulos KV, Fthenakis GC, Katsarou EI, Papadopoulos E (2021) Haemonchosis: A challenging parasitic infection of sheep and goats. Animals 11(2): 363.
- Fentahun T (2020) Systematic review on gastrointestinal helminths of domestic ruminants in Ethiopia. Online Journal of Animal and Feed Research 10(5): 216-230.
- Tariq KA (2015) A review of the epidemiology and control of gastrointestinal nematode infections of small ruminants. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences 85(2): 693-703.
© 2022 Kassahun Berhanu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






