- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
In Vitro Antimicrobial Susceptibility Testing of Azithromycin Against Bacteria Isolated from Patients with Respiratory Tract Infection
Ahmed Khalid A Siddig1, Khalid Saeed Hammad1, Leila Mohamed A Abdelgader1, Ghanem Mohammed Mahjaf1, Tibyan Abd Almajed Altaher2 and Mosab Nouraldein Mohammed Hamad3*
1Department of Medical Microbiology, Faculty of Medical Laboratory Sciences, Shendi University, Sudan
2Department of Clinical Chemistry, Faculty of Medical Laboratory Sciences, Shendi University, Sudan
3Assistant professor, Microbiology Department, Faculty of Medicine, Elsheikh Abdallah Elbadri University, Sudan
*Corresponding author: Mosab Nouraldein Mohammed Hamad, Assistant professor, Microbiology Department, Faculty of Medicine, Elsheikh Abdallah Elbadri University, Sudan
Submission: July 12, 2025;Published: September 10, 2025

ISSN 2578-0190 Volume7 issues 5
Abstract
Background: Azithromycin is an amoxicillin antibiotic that has been widely used in the treatment
of respiratory tract infections due to its broad-spectrum activity, favorable pharmacokinetics and
immunomodulatory properties.
Objective: To evaluate the in vitro sensitivity of common respiratory tract pathogens to Azithromycin.
Methodology: This is a prospective cross-sectional study conducted in Shendi City, Sudan, from January
to February 2025, at the Microbiology Laboratory, Faculty of Medical Laboratory Sciences at Shendi
University. A total of 30 sputum samples were collected, from which fourteen strains of pathogenic grampositive
bacteria and two strains of pathogenic gram-negative bacteria were isolated and identified using
Gram stain and biochemical tests.
Results: Of the 30 clinical specimens, Staphylococcus aureus was confirmed in 6 (60%), Streptococcus
pyogenes in 2 (20%), Klebsiella pneumoniae in 1 (10%) and Pseudomonas aeruginosa in 1 (10%).
Azithromycin demonstrated remarkable antimicrobial activity against all gram-positive bacteria and one
of the gram-negative bacteria (Pseudomonas aeruginosa), which showed intermediate sensitivity.
Conclusion: The findings of this study indicate that Azithromycin can be used as an antibacterial agent
against gram-positive bacterial strains.
Keywords: Antimicrobial; Azithromycin; Gram-positive bacteria; Respiratory tract infection
Introduction
Respiratory Tract Infections (RTIs) are among the leading causes of morbidity and mortality worldwide, significantly burdening healthcare systems [1]. These infections are often caused by bacterial pathogens, such as Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis, which are commonly associated with both upper and lower respiratory tract infections [2]. The widespread and often inappropriate use of antibiotics has led to the emergence of antimicrobial resistance, complicating the management of Respiratory Tract Infections (RTIs) and reducing the efficacy of commonly used antibiotics [3]. Is amacrolide antibiotic, has been widely utilized in the treatment of (RTIs) due to its broad-spectrum activity, favorable pharmacokinetics, and immunomodulatory properties [4]. However, increasing resistance to azithromycin among respiratory pathogens poses a challenge to the continued effectiveness of this medication. Periodic surveillance of local antimicrobial susceptibility patterns is essential to guide empirical therapy and ensure optimal clinical outcomes [5]. Although generally bacteriostatic, azithromycin is bactericidal against S. pyogenes, S. pneumonia and Haemophilus influenzae. Resistance appears to correlate with the amount of macrolide use within a community, as evidenced by a decrease in erythromycin resistance among group A streptococci associated with a nationwide decrease in macrolide use [6]. Azithromycin was developed for oral treatment of bacterial infections of the upper and lower respiratory tract caused by organisms such as S. pneumoniae and S. pyogenes, skin and skin structure infections caused by S. aureus and S. pyogenes [7]. In patients who are allergic to penicillins, erythromycin has been thought to be the primary treatment for Gram-positive streptococcal infections. Nonetheless, there have been reports of elevated erythromycin resistance in S. pyogenes isolates in a number of global locations [8,9]. The prevalence of resistance to other beta-lactams and other classes of antimicrobial agents has increased globally since S. pneumoniae developed penicillin resistance in the 1960s. This has led to clinical issues when treating infections caused by this microorganism and highlighted the need for alternative therapeutic approaches [10]. In vitro antimicrobial susceptibility testing of azithromycin for bacteria isolated from respiratory tract infections is crucial for guiding effective treatment, especially with rising resistance trends. It ensures appropriate empirical therapy, prevents treatment failures and supports personalized medicine.
Materials and Methods
This cross-sectional study was conducted in Shendi Locality, River Nile State, Sudan. Shendi is a historic town situated approximately 150km northeast of Khartoum on the east bank of the Nile and 45km southwest of the ancient city of Meroe. As the center of the Ja’aliin tribe and a traditional trading hub, the locality hosts multiple hospitals and clinical centers. Its principal suburb, Al-Matamma, lies on the west bank and connects to Northwest Sudan via a major trade route across the Bayuda Desert. The study population comprised patients presenting with symptoms of Respiratory Tract Infection (RTI). Inclusion criteria required participants to be admitted with RTI, while those under antimicrobial treatment were excluded. A total of thirty sputum samples (No=30) were collected from eligible patients. Data were obtained using a structured questionnaire documenting all study variables.
Collection of the specimens
Sputum was collected in sterile containers.
Cultivation of the specimens
Culture media, such as Chocolate agar, are used for the identification and isolation of clinical isolates.
Interpretation of cultural growth
The plates were observed for any bacterial colonies to grow significantly. The bacteria were well isolated and then identified by colonial morphology, Gram stain, and biochemical tests.
Preparation of bacterial suspension
Clinical isolates were isolated from different samples and subculture. Ten ml of normal saline was distributed in test tubes and sterilized in an autoclave at 121 °C for 15 minutes. A loopful of purified bacterium was inoculated into sterile normal saline. Inoculum density was compared with the McFarland standard solution.
Kirby-Bauer Disc Diffusion Method
The disk diffusion method (Kirby-Bauer technique) is a standardized approach for Antimicrobial Susceptibility Testing (AST). It involves applying an antibiotic. Impregnated disks to an agar plate inoculated with the test organism, and measuring inhibition zones are measured to determine susceptibility. Mueller-Hinton Agar (MHA) is the preferred medium due to its reproducibility and compliance with international guidelines [11].
Ethical consideration
Permission was given by the College Ethical Committee of SHENDI UNIVERSITY and Hospitals. Participants have been notified, and no coercion of any sort has been done and any information that may disclose the participant’s identity was not kept in consideration.
Data Collection and Analysis
A self-administered questionnaire was used and supported with coding numbers to facilitate the sorting of data. Data were entered, checked and analyzed using Microsoft Excel 2007. The final results were presented as frequencies and percentages.
Results
(Table 1-Table 7).
Table 1:The distribution of clinical specimens according to gender.
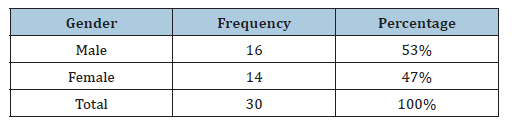
Table 2:The distribution of clinical specimens according to age.
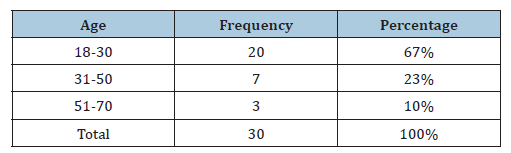
Table 3:The distribution of clinical specimens according to smoker.
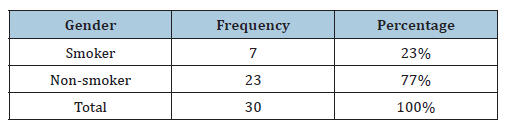
Table 4:Distribution of clinical specimens according to the type of smoking.
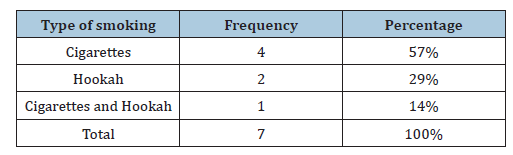
Table 5:Distribution of clinical specimens according to the rural-urban.
Table 6:Susceptibility of Gram-positive and Gramnegative bacteria to azithromycin./p>
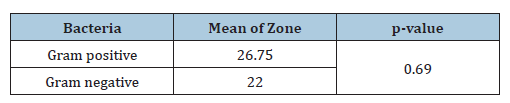
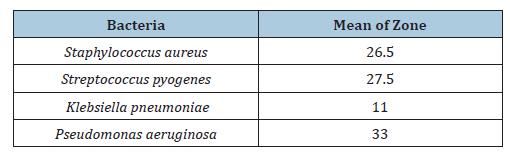
Table 7:Susceptibility of any type of Gram-positive and Gram-negative bacteria to azithromycin.
Discussion
The wide use of antibiotics in the treatment of bacterial infections has led to the emergence and spread of resistant strains and this has become a major cause of the failure of the treatment of infectious diseases [12]. The sensitivity of bacteria to antibiotics varies from place to place due to several factors, even within the same country or between different hospitals. 30 sputum samples were collected and many bacteria were isolated, like Staphylococcus and which represents 75% of the total Gram-positive bacteria isolated. And Streptococcus pyogenes, which represents 25% of the total gram-positive bacteria isolated, Staphylococcus and Streptococcus pyogenes together represent 80% of the total bacteria isolated. The remaining 20% of the total isolated bacteria were gram-negative bacteria, which included Klebsiella pneumoniae and Pseudomonas aeruginosa. Klebsiella pneumoniae represents 50% of the total Gram-negative bacteria and Pseudomonas aeruginosa also represents 50% of the remaining Gram-negative bacteria. Grampositive bacteria are the most susceptible to Azithromycin, which shows a mean zone of 26.75mm and gram-negative bacteria are less susceptible to Azithromycin, which shows a mean zone of 22mm. This study is consistent with the study by Javed which found that Staphylococcus aureus and Streptococcus pyogenes showed higher susceptibility to Azithromycin (mean zones: 25-28mm) compared to gram-negative bacteria like Klebsiella pneumonia (mean zone: 10-12mm) [11], Some studies found lower susceptibility in Grampositive bacteria Mishra reported that S. aureus had a mean zone of 22mm (compared to my result 26.5mm), suggesting variability in resistance patterns based on geographic location [13]. In Grampositive bacteria isolated Streptococcus pyogenes is the most susceptible to Azithromycin than S. aureus. Streptococcus pyogenes shows a mean zone of 27.5mm and Staphylococcus aureus shows a mean zone of 26.5mm. Altamimi’s studies show that Streptococcus pyogenes was more susceptible than Staphylococcus aureus. He reported that S. pyogenes had a mean inhibition zone of 28mm, while S. aureus showed 25mm [14] like my observation that Streptococcus pyogenes is slightly more sensitive. In gram-negative bacteria isolated Pseudomonas aeruginosa is susceptible to Azithromycin, which shows intermediate susceptibility with a mean zone of 33mm, and in Klebsiella pneumoniae, there is no susceptibility to Azithromycin, and it shows a resistant pattern, it is mean zone of 11mm. the research of Khan observed that Pseudomonas aeruginosa had a mean zone of 30-32mm, whereas Klebsiella pneumoniae was resistant (mean zone: 10mm) [15]. In Ahmed’s studies, Klebsiella pneumonia showed slight susceptibility, noting that some Klebsiella pneumonia strains had a mean inhibition zone of 15mm, indicating possible strain-dependent differences [16]. Pseudomonas resistance was higher in some reports. Patel found that Pseudomonas had a mean zone of 25mm, suggesting regional resistance variations [17]. Other studies agree with us by Leila Mohamed A. Abdelgader and her colleagues in 2024 found that out of the 19 S. pyogenes isolates that tested positive, only 12 (63.6%) were susceptible to azithromycin and just 7 (36.8%) were resistant [18]. Adequate preventive measures, such as teaching nursing staff to avoid as many nosocomial infections as possible, educating the public about the value of hygiene and motivating them to quit self-medicating and promoting closer scientific collaboration between clinicians and microbiologists, should be implemented in conjunction with antibiotic therapy to improve public health [18].
Conclusion
This study demonstrates that Azithromycin exhibits greater efficacy against gram-positive bacteria (such as Staphylococcus aureus and Streptococcus pyogenes) compared to gram-negative bacteria (including Klebsiella spp. and Pseudomonas spp.). Streptococcus pyogenes was found to be slightly more sensitive than Staphylococcus aureus, while Klebsiella showed notable resistance. Although some variations in susceptibility reported by other studies exist-potentially attributable to differences in bacterial strains or geographic locations-the overall findings consistently support Azithromycin being more effective for treating infections caused by gram-positive bacteria.
Recommendations
A. Test More Bacterial Strains: Check different strains of
Klebsiella and Pseudomonas to see if some respond better to
Azithromycin.
B. Compare Different Regions: Study bacteria from different areas
to understand why some show resistance while others don’t.
C. Use Other Antibiotics for Comparison: Test if other drugs work
better against resistant bacteria like Klebsiella.
D. Study Resistance Mechanisms: Find out why Gram-negative
bacteria resist Azithromycin more than Gram-positive ones.
E. Monitor Changing Resistance: Regularly test bacteria over time
to track if they become more or less resistant to Azithromycin.
Acknowledgment
Firstly, the praise be to Allah for providing us with strength to complete this study. Thanks to all staff of Medical Laboratories Sciences, Department of Medical Microbiology at Shendi University, Shendi-Sudan for helping complete this study.
Consent
The patient’s written consent has been collected.
Sources of Funding
There was no specific grant for this research from any funding organization in the public, private or nonprofit sectors.
Conflict of Interest
The authors have declared that no competing interests exist.
References
- Bartlett JG, Dowell SF, Mandell LA, File TM, Musher DM, et al. (2018) Respiratory tract infections: Pathogenesis, diagnosis and management. J Clin Microbiol 56(3): e01233-17.
- Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, et al. (2021) Pharmacokinetics and clinical utility of azithromycin in respiratory tract infections. Clin Infect Dis 72(5): 815-824.
- Gao Y, Lu X, Li T, Zhang Y, Chen Y (2022) Antimicrobial resistance trends in respiratory tract pathogens: A global perspective. Antimicrob Agents Chemother 66(1): e01521-21.
- Ventola CL (2015) The antibiotic resistance crisis: Part 1: Causes and threats. P T 40(4): 277-283.
- World Health Organization (2020) Global action plan on antimicrobial resistance. WHO, Geneva, Switzerland.
- Seppala H, Klaukka T, Varkila JV, Muotiala A, Helenius H, et al. (1997) The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med 337(7): 441-446.
- Hopkins S (1991) Clinical toleration and safety of azithromycin. Am J Med 91(3A): 40S-45S.
- Seppala H, Nissinen A, Jarvinem H, Huovinen S, Henriksson T, et al. (1992) Resistance to erythromycin in group A streptococci. N Engl J Med 326(5): 292-297.
- Betriu C, Redondo M, Palau ML, Sánchez A, Gómez M, et al. (2000) Comparative in vitro activities of linezolid, quinupristin-dalfopristin, moxifloxacin and trovafloxacin against erythromycin-susceptible and resistant streptococci. Antimicro Agents Chemother 44(7): 1838-1841.
- Jacobs MR, Appelbaum PC (2000) Susceptibility of 1100 Streptococcus pneumoniae strains isolated in 1997 from seven Latin American and Caribbean countries. Int J Antimicro Agents 16(1): 17-24.
- Koneman EW, Allen SD, Janda WM, Schreckenberger PC, Winn WC (2006) Color atlas and textbook of diagnostic microbiology. (6th edn), Lippincott Williams & Wilkins, Philadelphia, Pennsylvania.
- MacFaddin JF (2000) Biochemical tests for identification of medical bacteria. (3rd edn), Lippincott Williams & Wilkins, Philadelphia, Pennsylvania.
- Murray PR, Zeitinger JR (1983) Evaluation of Mueller-Hinton agar for disk diffusion susceptibility tests. J Clin Microbiol 18(5): 1269-1271.
- Cheesbrough M (2006) District laboratory practice in tropical countries. Part 2 (2nd edn), Cambridge University Press, New York, USA.
- Mahboob N, Iqbal H, Ahmed M, Magnet MH, Mamun KZ (2020) Disk diffusion method in enriched Mueller Hinton agar for determining susceptibility of candida isolates. J Dhaka Med Coll 28: 28-33.
- Ibrahim TA, Opawale BO, Oyinloye JM (2011) Antibacterial activity of herbal extracts against multidrug resistant of bacteria from clinical origin. Journal of Life Science Leaf Lets 15: 490-498.
- Javed H, Saleem S, Javed K (2018) Antibiotic susceptibility patterns of respiratory pathogens. J Infect Dev Ctries 12(5): 345-350.
- Abdelgader LM, Abdo TM, Mahjaf GM, Altaher TA, Hamad MN (2024) Antimicrobial Activity of azithromycin and erythromycin against Streptococcus Pyogenes isolated from sore throat patients in shendi, Sudan. SAR J Pathol Microbiol 5(1): 1-7.
© 2025, Mosab Nouraldein Mohammed Hamad. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






