- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Rare Case Study of Para-KDL with HIV Co-Infection and Their Treatment with Novel Combination Drugs Therapy Regimen
Anurag Pappu1, Major Madhukar1, Abhishek Kumar Rai2,3, Devendra Prasad Yadav2, Vahab Ali2,3,4* and Krishna Pandey1*
1Department of Clinical Medicine, ICMR-Rajendra Memorial Research Institute of Medical Sciences, Agamkuan, Patna-800007, INDIA
2Laboratory of Molecular Biochemistry and Cell Biology, Department of Biochemistry, ICMR-Rajendra Memorial Research Institute of Medical Sciences, Agamkuan, Patna-800007, INDIA
3Department of Zoology, University of Calcutta, Senate House, Kolkata-700073
4Faculty of Biological Sciences, Academy of Scientific and Innovative Research (AcSIR), Sector-19, Kamla Nehru Nagar, Ghaziabad-201002, U.P, India
*Corresponding author: Krishna Pandey, Department of Clinical Medicine, ICMRRajendra Memorial Research Institute of Medical Sciences, Agamkuan, Patna-800007, India Vahab Ali, Laboratory of Molecular Biochemistry and Cell Biology, Department of Biochemistry, ICMR-Rajendra Memorial Research Institute of Medical Sciences, Agamkuan, Patna-800007, Faculty of Biological Sciences, Academy of Scientific and Innovative Research (AcSIR), Sector-19, Kamla Nehru Nagar, Ghaziabad-201002, U P and Department of Zoology, University of Calcutta, Senate House, Kolkata-700073, India
Submission: May 12, 2025;Published: June 03, 2025

ISSN 2578-0190 Volume7 issues 4
Abstract
Visceral Leishmaniasis (VL), and Post Kala-Azar Dermal leishmaniasis (PKDL) are common problems in India, Nepal, Bangladesh, but both diseases can occur simultaneously sometime known as Para-Kala-Azar Dermal Leishmaniasis (Para-KDL). Similarly, HIV-VL co-infection is also common in Indian sub-continent. However, all three types of signs and symptoms occur rarely and have not yet been reported as per our knowledge. In September 2024 a rare case was diagnosed as VL-PKDL & HIV simultaneously in ICMRRMRIMS, Patna. The patient presented as fever with chills, loss of appetite, weakness and splenomegaly and mixed Nodulo-Papular lesions with positive LD bodies in the Splenic aspirates. Patient was also suffering from HIV-1 co-infection as confirmed by ELISA, low CD4+ level (176 cells/mm3) and HIV-RNA viral load (85 copies/ml). The patient was treated with six doses of Liposomal Amphotericin-B along with Miltefosine 50mg twice daily for 14 weeks. Follow-up studies after 5 months of treatment showed that patient was completely cured as signs and symptoms disappeared and he became healthy. Association of Para-KDL and HIV was noticed first time in Indian sub-continent and currently, no treatment guideline is available.
Keywords: Para-KDL; HIV; Liposomal amphotericin B resistance; Miltefosine
Introduction
Leishmaniasis is a parasitic disease caused by the protozoan parasite of the genus Leishmania which are transmitted to humans by the bite of a female Phlebotomine sand fly vector. The parasite resides in the insect vector as flagellated promastigotes and nonflagellated intracellular amastigotes in the mammalian host. Leishmaniasis impacts millions of people, particularly those from low socio-economic backgrounds, with an estimated annual incidence of 0.7 to 1.0 million new cases globally [1]. It is a vector borne disease caused by over 20 species of the Leishmania genus, and transmitted from humans to infected sand-fly vectors [2]. This disease manifests in various forms depending on the clinical symptoms and it varies from mild localized skin lesions cutaneous to more severe disfiguring mucocutaneous forms, and can even progress to a potentially life-threatening visceral form. Visceral Leishmaniasis is prevalent in 78 countries, with India, Nepal, Bangladesh, Sudan, and Brazil accounting for 67% of the worldwide VL burden [3,4]. Post-Kala-Azar Dermal Leishmaniasis (PKDL) is complication of VL that is typically seen in immuno-compromised individuals and
post treatment patients infected with L. donovani & L. infantum and it is characterized by macular, papular or nodular lesions, on the skin [5]. Approximately 5-10% of VL patients develop this dermal manifestation that is mainly associated with incomplete treatment. South East Asia (India, Nepal and Bangladesh) and East Africa (mainly Sudan) account for the majority of cases [6]. Some patients develop PKDL without having experienced any previous VL episodes and inadequate therapy also raises the risk of developing PKDL [7]. Usually, PKDL occurs as a separate event 2-3 years after the apparent cure of VL due to the reactivation of the dormant LD bodies on the dermis. The co-existence of VL and PKDL is not supported by their immunological mechanisms. However, in a rare instance, PKDL and active VL may co- exist. PKDL and active VL co-occurrence a condition called Para-kalaazar dermal leishmaniasis (Para-KDL). A number of symptoms, including fever, splenomegaly, hepatomegaly, or lymphadenopathy, as well as low nutritional status and the distinctive characteristics of PKDL rashes are indicative of Para-KDL. Para-KDL is similar to PKDL, but presents with symptoms of VL [8]. One of the biggest obstacles to Leishmania control is growing number of Leishmania- HIV co-infection cases that have been documented for VL and PKDL patients worldwide as a result of the AIDS pandemic’s expansion from urban to rural areas [9,10]. HIV-VL co- infection is now most prevalent in Southern Europe where upto 25-70% of adult patients of VL are co-infected with HIV [11]. Persons living with HIV have a higher risk of progression to VL after Leishmania infection, and face a poor prognosis once VL develops. Many patients with VLHIV co-infection fail to clear parasites from infected tissues and / or suffer from recurrent relapse [12]. However, the number of new Leishmania donovani-HIV co infected cases is rising in a few states of India, including Bihar and Jharkhand, which are endemic for VL [13]. Furthermore, treatment of VL-HIV is challenging, as patients face a higher risk of multiple relapses than non-HIV Patients. Thus, they are at much greater risk of death form the disease. HIV infected VL patients may become a significant source of ongoing transmission in the community due to their increased risk of high parasitic loads and relapses tendencies [14]. In addition to this, patients infected with HIV-VL co-infection along with PKDL remain infected at a sub-clinical level, increasing the challenge of a correct diagnosis and appropriate treatment and consequently restricting the control of a disease in endemic areas [15].
Case Presentation
A 60-year-old male patient from West Champaran district of Bihar was admitted in ward of Clinical Medicine division for the treatment to Rajendra Memorial Research Institute of Medical Science, Patna Bihar in September, 2024. He was suffering from fever with chills, nodular and papular lesions on the face, and very weak due to VL as well as HIV-co-infection. The fever was high grade (Highest recorded temperature was 102° F), intermittent in nature, and subsided by sweating. The patient also complained of substantial weight loss (underweight) due to having loss of appetite from 5 months. IgG based rK39 strip test was done which showed positive result. When we took detailed patient history, we found that this patient has previous history of VL 15 years back.
Clinical Diagnosis
Based on the positive result of rk39 strip test, we conducted hematological tests which revealed pancytopenia including mild hypo-chromia, mild anisocytosis and occasional tear drops (Table 1). Routine biochemistry tests in our institute revealed typical characteristic finding of hyper-gammaglobulinemia along with reversal of A-G ratio (Table 2) and other parameters were normal. We did physical examination of the patient which revealed left hypo-chondrium fullness due to splenic enlargement. Significant splenomegaly (6cm from the left coastal margin toward the right iliac fossa) was observed. However, we could not find hepatomegaly in this patient. We also found multiple mixed nodular, papular lesions present along with some hyper-pigmentation on dorsum, bilateral ala of nose and specifically some nodular lesion on upper part and philtrum ranging from 5.0mm to 2cm in diameter on upper lip (Figure 1a & 1c). Dermatological lesions developed gradually but remained non-itchy and non-tender, non-ulcerative, with no loss of sensation. Ultrasound abdomen analysis which revealed splenomegaly. Skin smear examination revealed 5+ LD bodies (Figure 2a) along with splenic aspirate analysis which revealed 2+ LD bodies in this patient (Figure 3a).
Table 1:Hematological study findings.
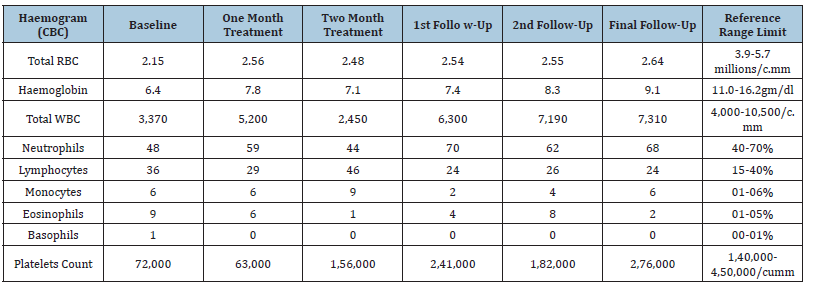
Table 2:Biochemistry investigation profiles.
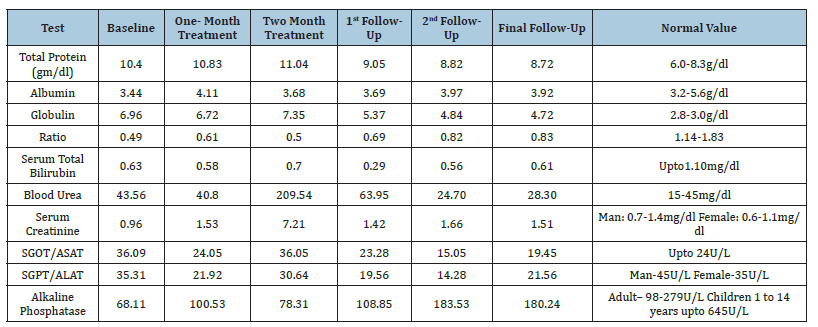
Figure 1:1a & 1c Patient with hyper pigmented and papular patches present on the nose, and nodular seen on philtrum part of upper lip respectively after treatment (1b & 1d) all lesions disappear.
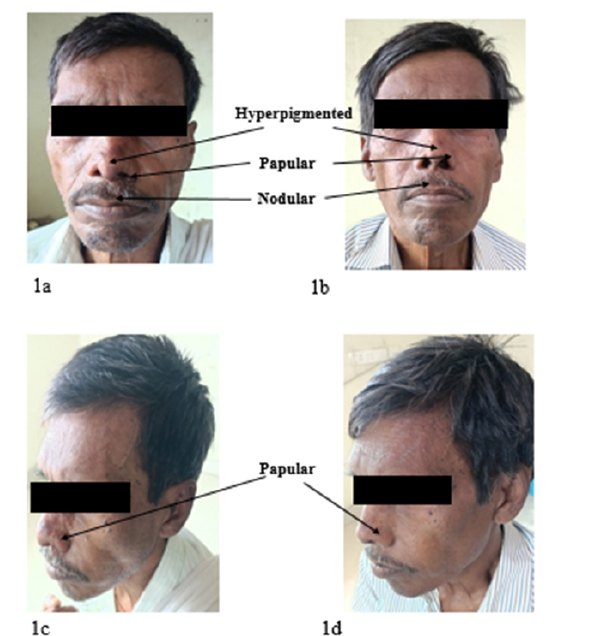
Figure 2:2a-Slit skin smear shows LD bodies before treatment respectively, LD bodies disappear (2b) After treatment.
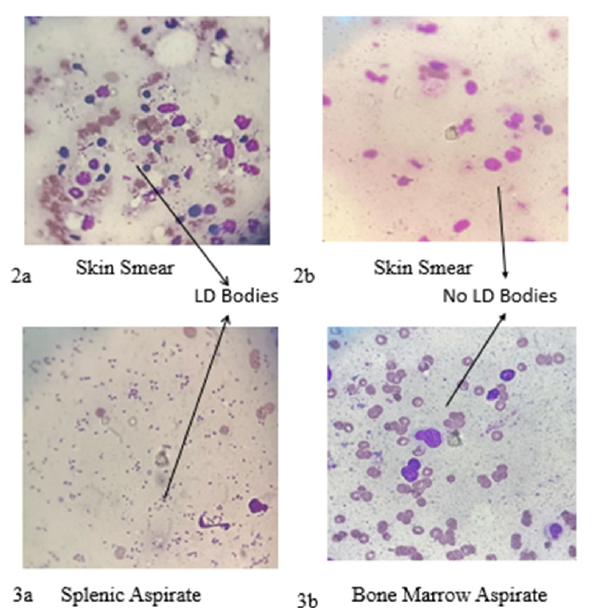
Figure 3:3a- Splenic aspirate shows LD bodies before treatment respectively, LD bodies disappear (3b) In bone marrow after treatment.

We also performed ELISA test for both HIV-1 & HIV-2 which shows only HIV-1 positive result along with VL & PKDL. Based on his past personal history records, he mentioned about unprotected sex practices with commercial sex workers, which led to acquisition of HIV-1 infection. His CD4+ count level was 176 cells/mm3 and HIVRNA viral load was 85 copies/ml, which confirmed HIV co-infection with VL & PKDL. On the basis of above findings and investigations, we demonstrated first time such a rare case of VL, PKDL and HIV co- infection simultaneously known as Para kala-azar dermal leishmaniasis (Para-KDL) from Bihar, India.
Treatment
The recommended treatment for the HIV-VL co-infected patient used is 6 dosage of Liposomal Amphotericin- B (5mg/kg body weight) along with Miltefosine 50mg twice a day for 2 weeks (14 days). However, the recommended treatment for the PKDL in immuno-competent patients involves a Miltefosine dosage of 50mg twice a day for 12 weeks (84 days) [16]. Because of the less sensitivity of the parasites as it resides in the skin macrophages or dendritic cells, treating PKDL necessitates a greater dose and longer duration, even though VL is more fatal [17]. In the case of this patient who suffered with VL, PKDL, and HIV co-infection, a treatment strategy was planned according to above mentioned guidelines with some modification. A case series was reported from our center (ICMR-RMRIMS), India and a case report from Bangladesh, where VL and PKDL in immunocompetent person was treated separately utilizing L-Amp-B for VL and Miltefosine for PKDL one month after the initial VL treatment [18,19]. Since, this rare case in immuno-compromised and has triple infection at a time so treatment is very challenging and required more attention. First, we treated HIV-VL co- infection with L-Amp-B (Intravenous) 5mg/ kg body weight (42kgx5=210mg) for 6 doses on alternate days as treatment strategy along with 50mg Miltefosine (Orally) twice daily for 2 weeks (14 days) was given.
However, we did not give gap in treatment strategy/regimen as previously reported for VL and PKDL infection simultaneously. We started treatment of PKDL just after the above-mentioned regimen for 84 days with miltefosine. In current scenario, there are no guidelines for the treatment of PKDL in immuno-compromised patients (HIV). After completion of treatment course for HIVVL co-infection and Miltefosine 50mg twice daily was started for next 12 weeks for treatment of PKDL. For the management of HIV, Anti-Retroviral Therapy (Tenofovir 300mg, Lamivudine 300mg, Dolutegravir 50mg once/day) was added just after the completion of HIV-VL and along with miltefosine twice daily 84 days for PKDL.
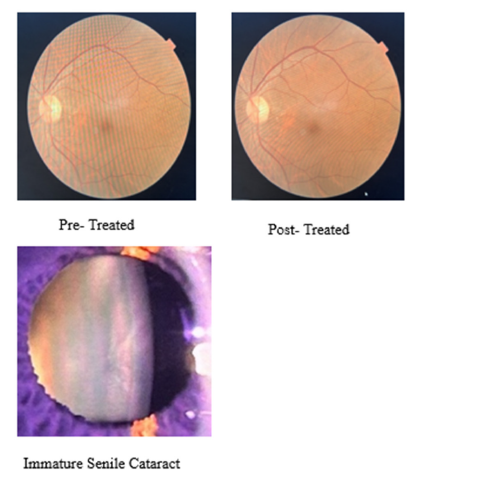
Major Events During Treatments
There is a guideline during treatment of PKDL with Miltefosine; ophthalmic examination is mandatory for follow up as standard operating protocol. In this case three Ophthalmic examinations were conducted (1) before the start of treatment, (2) One month of completion of treatment and (3) two months after completion of treatment. In this case after Ophthalmic Examination, we found very similar findings - Visual acuity-6/6, Cornea- Clear, Lens-Immature senile cataract (Age related), Optic disc- Normal, Macula- Normal etc., (Supplementary Figure 1). After completion of one and half month of treatment, patient come with complain of Fever, increase in frequency of burning micturition along with decrease in urine output for 12 days. So, we investigated this issue and found that this patient had Leucopenia (2450/microliter), and serum creatinine was raised (7.21mg/dl). Urine and blood culture was positive for E. coli. UTI induced AKI (Acute Kidney Injury), was diagnosed and it was treated with antibiotics, Alkalizers and sodium bicarbonate including miltefosine treatment. After complete treatment of Urinary Tract Infection (UTI) serum creatinine settled down to baseline level. Both urine and blood culture turned negative. Patient felt better without sign and symptoms of UTI. Bone marrow aspiration was negative (Figure 3b) after 15 days of treatment of HIV-VL co-infection. Skin biopsy was also negative (Figure 2b) at one month after treatment completion of PKDL.
Supplementary Figure 1:Ophthalmic Examination.
Findings After Five Months of Complete Treatment
Follow-up study of this patient, after 5 months of complete treatment, revealed that he was afebrile, general condition had improved, appetite was better, spleen size reduced and was non palpable. Haemoglobin improved up to 9.1gm/dl, total WBC count 7,310/microliter and thrombocyte 2,76,000/microliter. All nodular- popular & hyper pigmented lesions disappeared from dorsum of nose and upper lip (Figure 1b & 1d).
Treatment
L-Amp-B 30mg/kg in 6 divided doses along with Capsule Miltefosine 50mg twice daily for 14 weeks was given for treatment of HIV and Para-KDL co infection.
Conclusion
This is first case report of concomitant Para-KDL in HIV infection in Bihar India. We had diagnosed and treated such triple VL-PKDL & HIV co-infected case successfully which gives further insight for diagnosis of Para-KDL with HIV and new strategy for treatment. Recently, India has been declared the country to eliminate kala-azar, with an elimination target of less than 1 case/10,000 population at sub district level. But it cannot be sustained unless all sources of infection are treated successfully such as HIV-VL and PKDL.
Consent for publication
Written informed consent was obtained from the participant for publication of the case report.
References
- WHO (2023) On-line Key information about leishmaniasis.
- Burza S, Croft SL, Boelaert M (2018) Leishmaniasis. Lancet 392(10151): 951-970.
- Alvar J, Boer MD, Dagne DA (2021) Towards the elimination of visceral leishmaniasis as a public health problem in East Africa: Reflection on an enhance control strategy and a call for action. Lancet Global Health 9(12): 1763-1769.
- Chappuis FO, Sunder S, Hailu A, Ghalib H, Rijal S, et al. (2007) Visceral leishmaniasis what are the needs for diagnosis, treatment and control? Nat Rev Microbiol 5 (11): 873-882.
- Zijlstra EE (2016) The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasites Vectors 23(9): 464.
- Jafarzadeh A, Jafardeh S, Sharifi I, Aminizadeh N, Nozari P, et al. (2021) The importance of T cell- derived cytokines in post-kala-azar dermal leishmaniasis. Cytokine 147: 155321.
- Ghosh P, Roy P, Chaudhuri SJ, Das NK (2021) Epidemiology of post-kala-azar dermal leishmaniasis. Indian J Dermatol 66(1): 12-23.
- Zijlstra EE (2014) PKDL and other dermal Leisons in HIV co-infected patients with leishmaniasis: Review of clinical presentation in relation to immune response. Plos Neglected Tropical Disease 8(11): e3258.
- Alvar J, Canavate C, Gutierrez-Solar B, Jimenez M, Laguna F, et al. (1997) Leishmania and human immunodeficiency virus co-infection: The first 10 years. Clin Microbiol Rev 10(2): 298-319.
- Lindoso JA, Cota GF, Cruz AMD, Goto H, Maia-Elkhoury AN, et al. (2014) Visceral leishmaniasis and HIV coinfection in Latin America. PLoS Negl Trop Dis 8(9): 3136.
- Barth-Jaeggi T, Maser P (2021) Leishmaniasis in Europe and Central Asia: Epidemiology, impact of habitat and lifestyle changes, HIV co-infection. In: Steinmann P, Utzinger J (Eds), Neglected tropical diseases- Europe and Central Asia. Cham, Springer International Publishing, Switzerland, pp. 83-99.
- Takele Y, Mulaw T, Adem E (2020) Recurrent visceral leishmaniasis relapse in HIV co-infected with visceral leishmaniasis and HIV; a qualitative study from Bihar, India. Plos One 15: e0227911.
- (2024) National AIDS Council Organization. Operational guidelines.
- Cloots K, Marino P, Burza S, Gill N, Boelaert M, et al. (2021) Visceral leishmaniasis-HIV co-infection as a predictor of increased leishmania transmission at the village level in Bihar, Inida. Front Cell Infect Microbial 11: 604117.
- Akhoundi M, Downing T, Votýpka J, Kuhls K, Lukeš J, et al. (2017) Leishmania infections: Molecular targets and diagnosis. Mol Asp Med 57: 1-29.
- Burza S, Mahajan R, Kazmi S, Alexander N, Kumar D, et al. (2022) AmBisome monotherapy and combination ambisome- miltefosine therapy for the treatment of visceral leishmaniasis in patients co-infected with immnuno deficiency virus in India: A randomized open -label, parallel-arm, phase-3 trial. Clinical Infectious Disease 75(8):1423-1432.
- Saravolatz LD, Bern C, Adler-Moore J, Berenguer J, Boelaert M, et al. (2006) Liposomal amphotericin B for the treatment of visceral leishmaniasis. Clinical Infectious Disease 43(7): 917-924.
- Kumar R, Rabi Das V, Topno RK, Pal B, Imam A, et al. (2016) Para-kala-azar dermal leishmaniasis cases in Indian subcontinent- A case series. Pathogen and Global Health 110(7-8): 326-329.
- Hasan Md, Proma BS, Hossain Md, Arifuzzaman Md, Islam N, et al. (2023) A case report on para-kala-azar dermal leishmaniasis: An unsolved mystery. BMC Infectious Disease 23(1): 885.
© 2025, Krishna Pandey And Vahab Ali. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






