- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Disinfection of Mycotic Species Isolated from Cases of Bovine Mastitis Showing Antifungal Resistance
Elaine Meade1, Micheal Savage2, Mark Anthony Slattery3 and Mary Garvey1,2,3*
1Department of Life Science, Ireland
2Lir Analytical LTD, Ireland
3Mark Anthony Slattery, Ireland
*Corresponding author: Mary Garvey, Department of Life Science, Ireland
Submission: March 04, 2020; Published: March 20, 2020

ISSN 2578-0190 Volume3 issues5
Abstract
Fungal disease has emerged as a major medical problem with resistance to the four classes of antifungal agents a common factor promoting fungal virulence. Mycotic infection has a high mortality rate in immunocompromised persons as prolonged aggressive colonisation occurs. As such the medical and veterinary importance of recalcitrant fungal disease is undeniable. Studies described herein detail fungal species associated with bovine mastitis, resistance levels and investigate biocidal control options for use in situ. Effective biocidal options for prophylactic disinfection are suggested to limit fungal transmission with the overall aim of protecting animal health and the food chain.
Keywords: Mycotic; Mastitis; Resistant; Biocidal Efficacy; Protect; Dairy
Introduction
Bovine mastitis represents a serious and persistent problem for the dairy industry with reduced milk yield, economic costs and often severe consequences for diseased animals. Typically, clinical and subclinical forms are treated with antibiotics as bacterial species are frequently suspected as the causative agent of the intramammary infections (IMIs). Unsuccessful antibiotic therapy, however, leads to continued morbidity and horizontal pathogen transmission within herds with the proliferation of antimicrobial resistance (AMR) an associated issue. Furthermore, persistent non-responsive IMIs often leads to culling of dairy cows in an attempt at curbing disease transmission. Mycotic species are emerging globally as the etiological agents of disease particularly in epizootic hard to treat cases. Fungal infections are hard to recognise, difficult to treat and often chronic in nature. Indeed, the use of antibiotic and corticotherapy which are recommended for IMIs both prophylactically and curatively may lead to mycotic proliferation within the mammary gland [1]. Studies report mycotic IMIs resulting from Candida species colonising the bovine mammary gland, where they are considered opportunistic pathogens having the ability to use antibiotic drugs as nutrient sources, proliferating their growth [2]. Candida albicans, non-Candida albicans species, Trichospron and Cryptococcus neoformans are recognised commensal and environmental pathogens associated with bovine mastitis. Candida and Cryptococcus are important zoonotic species imperative for public health safety as they lead to invasive fungal infections in humans causing serious morbidity and mortality. The elimination of antagonistic bacterial species via antibiotic treatment encourages mycotic reproduction with an influx of immune cells to the udder potentially leading to systemic fungemia. This dysbiosis within the mammary gland may promote clinical symptoms and negatively impact milk quality and yield. Mycotic mastitis resulting from fungal infection is correlated with poor environmental and fomite cleanliness, dirty bedding, contaminated therapy solutions, seasonality and humid temperatures [3]. Fungal virulence factors such as biofilm formation, secretion of aspartyl proteinases, haemolysin production, spore formation and favourable temperature requirements play a significant role in their pathogenicity. Fungal enzymes enable the invasion of tissues from the mammary gland and allows for the development of systemic infections. Mycotoxins, a group of highly toxic compounds produced by fungal species including ochratoxin, zearalenone and fumonisins have been identified in bovine milk [4]. Antifungal drug therapy has been used for mycotic mastitis but there is a lack of evidence relating to drug efficacy and fungal and yeast resistance [3]. As public awareness of food safety increases the recognition of zoonotic pathogens as contributors to global incidence of disease is a key topic of concern. Indeed, as many as half a million people suffer from candidiasis worldwide, with a staggering annual mortality rate of 45-75% [5]. Prophylactically protecting bovine health using adequate environmentally friendly disinfectants is key to safeguarding animal and human health, a key component of “One Health”. An effective prevention and control strategy including udder hygiene, environmental disinfection and milking machine sanitisation is required to reduce and eliminate disease within herds while also protecting the food chain. This study reports on the antifungal resistance of mycotic species isolated from bovine cases of mastitis where animals were non-responsive to antibiotic therapy and subsequently culled. Bacterial isolates from each case which were assessed for susceptibility and resistance to veterinary prescribed antibiotic agents, previously reported by Meade et al. [6] where no resistance to the treatment regime was evident bacterial species. Effective antifungal biocide solutions are also identified for application in animal housing and milking parlour environments. Pathogen dissemination and proliferation can be controlled by proper disinfection of housing areas, milking machines and equipment. Such measures have reduced the risk of bacterial environmental and contagious mastitis and show potential at controlling fungal transmission.
Methods
The following research presented is in continuation of ongoing studies conducted on various microbial pathogens isolated from dairy cows suffering with chronic cases of IMI post-partum. Previously bacterial species isolated, including S. Uberis, B. cereus, S. aureus, A. buamannii, P. aeruginosa and E. coli, were assessed and reported on [6]. In many cases of chronic mastitis, culling of the animal from the herd is not uncommon, where persistent symptoms often leave farmers no other option. This scenario can be seen in prior cases reported, where patients continued to remain unresponsive to antibiotic treatment. Therefore, further studies on isolated pathogenic fungal species were carried out. In addition to previously reported cases, a further case investigated included a 7-year-old Holstein/British Friesian cross lactating dairy cow presenting with inflamed, chronic mastitis 19 days post-partum. Clinical symptoms of heat, swelling, redness and pain were apparent in two front affected quarters where milk consumption was watery with clots present. Patient initially prescribed IM dexamethasone (2mg/ml) at a dose of 1.5mL per 50kg for 10 days. Dexamethasone was further prescribed 3 weeks later at the same dose, in combination with Tylosin (10mg per kg per day) for 5 days, where IM injection of procaine benzyl penicillin and dihydrostreptomycin (4ml per 100kg bodyweight) had also been used intermittently on two separate occasions for 3 consecutive days at a time to help treat symptoms. Cow remained unresponsive to treatment and was culled from the herd. Microbial analysis identified various pathogenic Gram-negative and Gram-positive species as well as fungal pathogens, Candida krusei and Candida tropicalis, where the total viable count (TVC) was 8.83E+04TVC/mL. Pathogenic species isolated and used for this study were obtained from all reported cases and are as follows Candida albicans, Candida krusei, Candida tropicalis, Cryptococcus neoformans, Trichosporon lactis and Wickerhamomyces anomalous.
Microbial isolation and identification
Collected samples of intramammary infection were inoculated in sabouraud dextrose broth (Cruinn Diagnostics, Dublin, Ireland) and incubated under rotary conditions (125rpm) at varying temperatures of 25 ℃, 30 ℃ and 37 ℃ respectively for up to 72hours, streaking intermittently onto sabouraud dextrose agar (Cruinn Diagnostics, Dublin, Ireland). Fungal total viable cell counts were performed by diluting milk samples in sterile phosphate buffered saline (PBS) with standard plate counts performed after 48-72 hours incubation. Individual colonies were re-streaked for isolation and pure isolated colonies inoculated into sabouraud broth for further biochemical characterization. Colonies were identified based on their morphological characteristics, biochemical profile and growth on selective agars, specifically CHROMagar™ Candida (CHROMagar, Paris) and HiMedia™ Cryptococcus Differential Agar. Identity was confirmed via colony polymerase chain reaction (PCR). Specifically, a single colony of each fungal isolate was picked from a 48-hour culture using a sterile micropipette tip and suspended in 100µl sterile deionized water. Fungal primers ITS1-F 5’-CTT GGT CAT TTA GAG GAA GTA A-3’ and ITS4 5’-TCCTCCGCTTATTGATATGC-3’ (Sigma Aldrich, Dublin, Ireland) were used for direct amplification of intergenic spacer regions (ITS) of rDNA. Direct colony PCR was performed in a total reaction volume of 20µl, containing 17µl red Taq 1.1x master mix (VWR, Dublin, Ireland) 1µl ITS1F, 1µl ITS4 and 1µl of selected colony suspension. DNA amplification was performed in a thermo cycler (VWR, Dublin, Ireland) using the recommended parameters. Clean-up and gene sequencing of PCR products was completed by Source Bioscience (Waterford, Ireland). Strains were stored and cultured in sabouraud broth/agar (at 30 ℃ for Candida spp., Cryptococcus neoformans and Trichosporon lactis, and 25 ℃ for Wickerhamomyces anomalous) and identity confirmed via Gram stain prior to each experimental set up.
Kirby Bauer disk diffusion assay using antifungal drug therapy
Antifungal susceptibility patterns of isolated mastitic strains to amphotericin (Amp B), caspofungin and fluconazole (Sigma Aldrich, Dublin, Ireland) were assessed via disc diffusion method. Three reference control strains, Candida krusei ATCC 14243, Candida albicans ATCC 10231 and Cryptococcus neoformans ATCC 32045 were included for quality control and sensitivity analysis to disease isolates. In addition, the effect of DMSO on fungal growth was evaluated by testing the different concentrations without the antifungal agent to negate the effect of DMSO induced toxicity, as it was used for drug dissolution. The Kirby Bauer assay was conducted as per Meade et al. [7,8] with zones of inhibition measured in millimetre (mm) following 48 hours incubation at 30 oC for Candida spp., C. neoformans and T. lactis, and 72 hours incubation for W. anomalous at 25 oC. Concentrations tested ranged from 2.5 to 50µg/mL for Amp B, 2.5-200µg/mL for caspofungin and 2.5-250µg/mL for fluconazole.
Pasteurisation
Flash pasteurization or high-temperature short-time (HTST) pasteurization is a commonly used standard heat method in which perishable beverages such as milk is subject to high temperature for a short time in order to kill spoilage microorganisms, to allow for extended unrefrigerated storage and to retain flavour. Specifically, the pasteurization process was conducted as per Meade et al. [6], whereby 1mL of microbial suspension containing 1x10⁷cfu/ml was transferred to 9ml sterile full fat milk. The resulting suspensions were then heated to 72 °C for 15 secs followed by rapid cooling to 4 °C. Subsequently, the test solution was 10-fold serially diluted and 500µl aseptically spread on sabouraud dextrose agar plates in triplicate. All plates were inverted and incubated at 30 °C for Candida spp. for 48 hours, 30 °C T. lactis and C. neoformans for 72 hours and a 5-day incubation period at 25 °C for W. anomalous. Surviving colonies were counted and reported as log10 cfu/ml compared to an untreated control. Antifungal resistance was re-assessed after the pasteurization process to determine if heat treatment impacts on the levels of resistance of isolates.
Novel biocidal options
The antimicrobial agents investigated in this study are pure biocides used as disinfectants alone or in commercial brands. The concentrations described are the concentration of the active component and include the concentration used following manufactures instructions. Test solutions studied for use in veterinary areas and as farm disinfectants utilise peracetic acid or triameen as active ingredients. Peracetic acid is a powerful oxidant capable of oxidising the outer cell membranes of micro-organisms, acting as a biocidal disinfectant. Triameen is a fatty amine derivative and a highly effective antimicrobial agent. Studies by Meade et al. [6] have demonstrated the antimicrobial potential of both disinfectants on reference strains sourced from the American Type Culture Collection (ATCC) bank. In the present study, the activity of both biocides will be determined against multidrug resistant fungal pathogens isolated from incidents of mastitis in dairy cows where mortality was the end result in some cases.
Kirby Bauer disk diffusion assay using novel biocidal solutions
The Kirby Bauer assay was carried out on all test isolates to determine the effect of disinfectants on microbial species with the presence and absence of an interfering substance. 1mL of ca. 1x10⁷ microbial cells was added to 9mL BSA to give a working microbial count of 10⁶ cells in solution with 3g/L or 10g/L BSA and with 10g/L yeast extract (YE) (Sigma, Ireland) as low- and high-level interfering substance. Subsequently, 100μL 10⁶ cells/mL microbial suspension were transferred onto replicate agar plates and spread with a sterile L-shaped spreader (Cruinn Diagnostics) to ensure even disruption across the agar surface. Filter disks (6mm) were immersed in the test biocide solutions at concentrations of 0.01,0.1 and 1% (v/v) for 15 seconds and excess solution was al-lowed to drip off the disk. Subsequently, the disk was placed on the inoculated plate. Plates were then incubated for the required time period and temperature. Zones of inhibition were then measured using a Vernier calliper in millimetres as per Meade et al. [7], for each test chemical and each test organism.
Antifungal activity suspension test BSEN 1650
Suspension tests were conducted as per the methods of the European guidelines for fungicidal or yeasticidal testing of chemical disinfectants that form a homogeneous physically stable preparation in hard water for use in in food, industrial, domestic and institutional areas. Isolated strains from the all described cases of mastitis were used for this suspension test. Test Candida species were cultured for 16 hours (C. neoformans and T. lactis required 48-hour culture period while W. anomalous required a 5-day culture period), after which cell counts were adjusted to 107cfu/ml with sterile PBS. Chemical test solutions were prepared as per manufacture instructions for use on site and at a concentration above and below this working concentration giving a range of 0.01,0.1 and 1% (v/v). Prior to testing all reagents are equilibrated to the test temperature of 20 °C using a water bath. Subsequently 8 ml of the test product was transferred to a sterile container with 1ml of sterile water. Afterward, 1ml of microbial suspension containing 1x107 fungal cells was added. Additionally, 1ml of interfering substance at 3.0g/L BSA (low level soiling) and 10g/L BSA with 10g/L YE (high level soiling) was added with subsequent incubation for 0 to 15 minutes with mixing in a 20 °C water bath. At set intervals of 5,10 and 15 minutes 1ml of the test mixture was transferred into a tube containing 8ml neutralizer and 1ml of sterile water. Samples were mixed and incubated in the water bath for 5 minutes. After neutralization with 30g/l polysorbate 80+3g/l lecithin/l-a-phosphatidylcholine from egg yolk (Sigma, Ireland), 100µl of this bacterial suspension was transferred onto agar plates in triplicate and incubated at 30 °C for 48 hours for Candida spp, 30 °C for 72 hours for C. neoformans and T. lactis and a 5 day incubation period for W. anomalous at 25 °C. Surviving cells of treated organisms was counted to determine the level of fungal inactivation following exposure to the test solutions compared to the untreated control (PBS). For compliance with this test, test chemicals must achieve a 104 fungal cell reduction in treatment times less than 30 minutes.
Statistics
All the experiments were performed three times with three plate replicates for each experimental data point providing a mean result for each experimental batch. Average zone diameter was calculated for the Kirby Bauer assay with standard deviation and significance levels at 95% confidence determined for each strain. For suspension testing the log10 reduction was calculated as the log reduction in viable cell numbers (cfu/ml) of the non-treated (N0) and treated (N) samples [log10 (N0/N)]. Student T tests were conducted to determine significance levels (p<0.05) of fungal susceptibility to treatment and levels of susceptibility or resistance between species investigated using Minitab 16 (Minitab Ltd, Coventry, UK).
Results
Table 1 shows antifungal susceptibility of isolated species Candida albicans, Candida krusei, Candida tropicalis, Cryptococcus neoformans, Trichosporon lactis and Wickerhamomyces anomalus to antifungal therapeutic agents. Amphotericin B provided zones of inhibition for all strains with the most sensitive strain being T. lactic followed by C. tropicalis, C. neoformans, W. anomalus, C. krusei and C. albicans at 50µg/ml. At concentrations exceeding this no increase in zone diameter was achieved for Amphotericin B. Quality control ATTC strains of C. albicans, C. krusei and C. neoformans proved more sensitive to Amp B than their isolated counterparts at 50µg/ml. Where a zone diameter of 14,8 and 15mm was obtained for C. albicans (ATCC 19231), C. krusei (ATCC 14243) and C. neoformans (ATCC 10231) (data not shown) compared to 6.3,7 and 10mm for the isolated counterparts (Table 1). C. albicans, C. tropicalis and T. lactic demonstrate clear resistance to fluconazole at concentrations of up to 200µg/ml. C. neoformans displayed the greatest sensitivity (8.7mm) to fluconazole followed by C. krusei and W. anomalus with optimal inhibition at 200µg/ml. Fluconazole provided zones of inhibition of 0,7 and 18mm for control strains of C. albicans (ATCC 10231) and C. krusei (ATCC 14243) and C. neoformans (ATCC 32045) respectively, demonstrating drug efficacy for non-isolated species (data not shown) with the exception of C. albicans ATCC. T. lactic displayed resistance to caspofungin up to 200µg/ml with this concentration giving a zone diameter of 6.1mm only. All other test isolates show sensitivity to caspofungin with optimal inhibition obtained at 200µg/ml, where a zone diameter of 21.5,21,12.5,8,8 and 6.1mm was obtained for C. krusei, C. neoformans, C. albicans, C. tropicalis, W. anomalus and T. lactic respectively. A diameter of 20,21 and 5mm was obtained for 200µg/ml caspofungin on control ATCC strains of C. albicans, C. krusei and C. neoformans respectively suggesting increased sensitivity in isolated species. As with fluconazole concentrations exceeding 200µg/ml did not provide an increase in zone diameter for any strain tested.
Table 1: Zones of inhibition (mm) produced by licensed antifungal agents against zoonotic fungal isolates from mastitis cases in dairy (+/- Standard deviation).
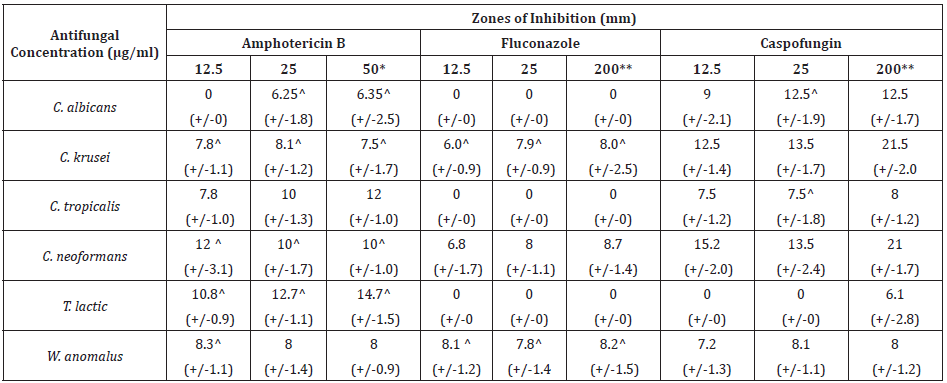
*No increase in zone diameter was achieved with concentrations of AMP B exceeding 50μg/ml. **No increase in zone diameter was achieved with concentrations of fluconazole and caspofungin exceeding 200μg/ml. ^indicates test species which showed statistically significant decreased zones of diameter post-pasteurisation compared to pre-pasteurisation
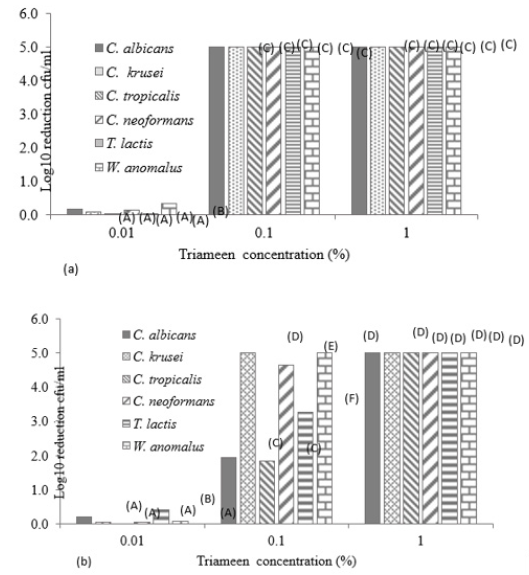
Figure 1 displays the viable and non-viable cell numbers of all test isolates pre and post flash pasteurisation. For all test species, significantly high numbers remained viable post treatment with the order of decreasing survivability as follows: T. lactic, W. anomalus, C. krusei, C. tropicalis, C. albicans and C. neoformans. C. neoformans proved most sensitive to flash pasteurisation with a 3.3 log10cfu/ml death of treated cells compared to the 1.5 log10cfu/ml death achieved for T. lactic. Interestingly, an increase in resistance to Amp B was observed in T. lactic following pasteurisation (Table 1) evident as decreased zone diameters. A zone diameter of 14.7mm pre pasteurisation and 4.75mm post pasteurisation for T. lactic was obtained at 50µg/ml Amp B. This post heat treatment increase in resistance to Amp B was also observed for C. albicans, C. krusei and C. neoformans (complete resistance emerged). C. krusei and W. anomalus also demonstrated increased resistance to fluconazole post flash pasteurisation. This constitutes an important find as all fungal isolates show clear resistance to heat treatment with increased antifungal resistance also present in certain treated species. All isolated strains demonstrate susceptibility to peracetic acid at 1% with zones exceeding that of the control chemical 70% iso propyl alcohol with and without the interfering agent BSA (Figure 2). At all concentrations of peracetic acid, W. anomalus proved consistently the most sensitive strain tested with a zone of inhibition of 40mm for all concentrations of BSA. This reduced to ca. 23mm at 0.1% peracetic acid for this strain. C. albicans proved the most resistant strain with zones of inhibition of ca. 18mm followed by C. tropicalis. IP70% provided consistent zones not exceeding 8mm for all species regardless of the presence of BSA. The lowest concentration of peracetic acid (0.01%) provided similar fungal inhibition to the IP70% for C. krusei, C. tropicalis, T. lactic and W. anomalus (Figure 2a) in the absence of BSA with no inhibition of C. albicans evident. The presence of the interfering substance BSA at 3 and 10g/L does appear to affect the effectiveness of peracetic acid at 0.01% but not at concentrations exceeding this. Compared to peracetic acid, triameen demonstrated less efficacy at inhibiting fungal growth for all species (Figure 3) with T. lactic the most sensitive strain at 1% concentration. C. tropicalis proved the most resistant strain followed by C. albicans. As with peracetic acid, 0.01% triameen provided similar levels of inhibition to IPA70% irrespective of the presence of BSA. C. albicans did not display resistance to triameen at this lower concentration as observed with peracetic acid. While triameen appears less effective than peracetic acid (demonstrated by reduced zones of inhibition) it appears more effective at the lower concentrations tested and is not inhibited by the presence of the interfering substance. Comparative ATCC strains of C. albicans, C. krusei and C. neoformans proved significantly more sensitive to both biocidal options in the absence and presence of interfering substance. At the highest concentration of 1% peracetic acid in high level soiling conditions a zone of inhibition of 25,24 and 30mm was obtained, with 17.5, 23.5 and 30mm obtained for 1% triameen for ATCC C. albicans, C. krusei and C. neoformans respectively (data not shown). Figure 4 displays the BSEN 1650 biocidal testing of all isolates for peracetic acid where only the 1% concentration provided the necessary 4 log10cfu/ml loss of viability for test strains in both 3 and 10g/L interfering agent in 15 minutes. C. albicans again appears the most resistant to peracetic acid where a maximal 3.2 log10cfu/ml inactivation was obtained at the highest concentration tested of 1% in 15 minutes increasing to the required 4 log10cfu/ml at 30 minutes (data not shown). Triameen appears more effective in this assay with a 4 log10cfu/ml loss in viability of all test strains obtained for 0.1% in the presence of 3g/L BSA in 15 minutes (Figure 5a). A decrease in this effectiveness was observed at the higher concentration of BSA for C. albicans, C. tropicalis and C. neoformans (Figure 5b) suggesting that the presence of organic matter may impede its biocidal efficacy for these strains. Peracetic acid is potent enough to provide the 4log10cfu/ml loss in viability at 1% concentration in both low level (3g/L BSA) and high-level soiling (10g/L BSA and YE) in under 15 minutes. Triameen at 0.1% is potent enough to meet the BSEN requirements in low level soiling environments at 0.1 and at 1% in 15 minutes with 30 minutes needed in high level soiling environments at these concentrations.
Figure 1: Viable and non-viable cell numbers (log10 cfu/ml) (+/- S.D) of zoonotic fungal isolated species post flash pasteurisation of 6 log10 cfu/ml viable cells at 72 ℃ for 15 seconds with rapid cooling to 4 ℃. A, B,C,D,E,F,G and H denotes significant difference at p>0.05.

Figure 2: Zones of inhibition (mm) of peracetic acid and 70% IP for mastitis isolated fungal species (a) in the absence of BSA, (b) presence of 3g/L BSA and (c) 10g/L BSA with 10g/L yeast extract (+/-S.D.).

Figure 3: Zones of inhibition (mm) of triameen and 70% IP for mastitis isolated fungal species (a) in the absence of BSA, (b) presence of 3g/L BSA and (c) 10g/L BSA with 10g/L yeast extract (+/-S.D.).
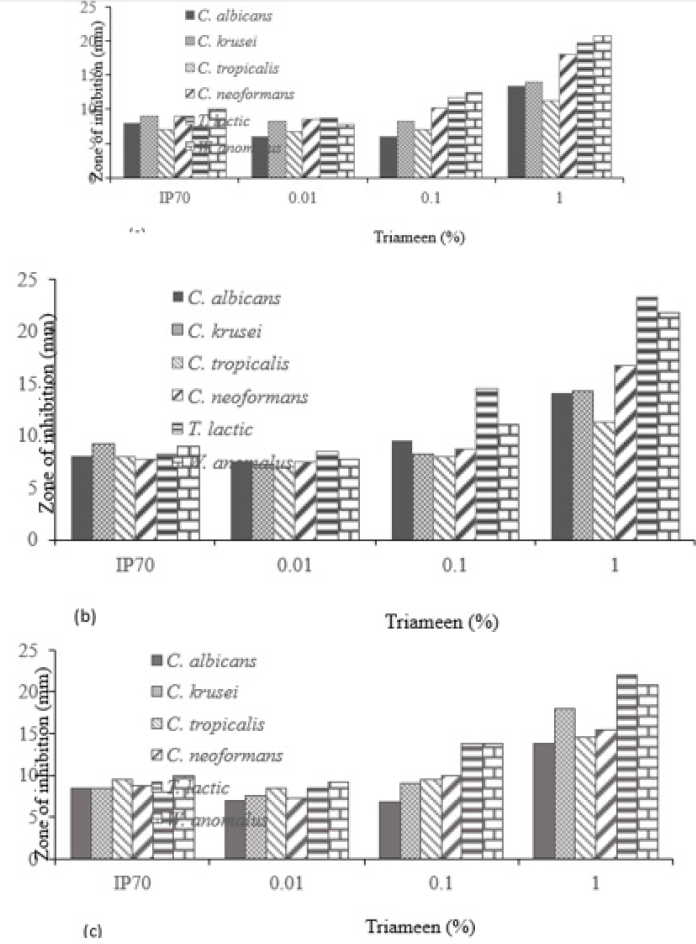
Figure 4: Log10 reduction in cell viability (cfu/ml) of mastitis isolated fungal species following exposure to varying concentrations of peracetic acid in the presence of interfering agent a) 3g/L BSA and b) 10g/L BSA and 10g/L yeast extract in accordance with BSEN 1650. Data obtained for 15 minutes treatment time (+/- S.D).
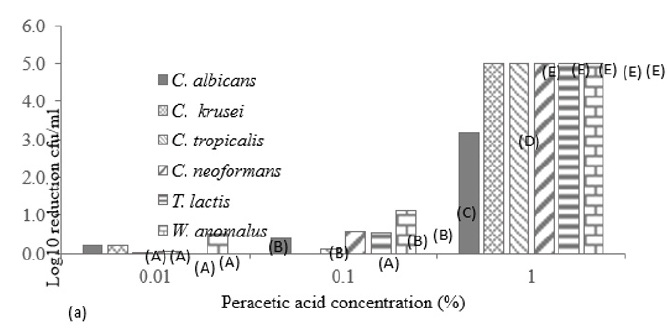
Figure 5: Log10 reduction in cell viability (cfu/ml) of mastitis isolated fungal species following exposure to varying concentrations of triameen in the presence of interfering agent a) 3g/L BSA and b) 10g/L BSA and 10g/L yeast extract in accordance with BSEN 1650. Data obtained for 15 minutes treatment time (+/- S.D). A,B,C,D,E and F denotes significant difference at p>0.05.
Discussion
Bovine mastitis remains and ongoing problem globally with economical costs and yield losses at production level and animal mortality at herd level. Small dairy holders are particularly impacted by the financial costs of culling animals. Morbidity is more frequent in chronic cases of mycotic mastitis than acute with the excessive use of antibiotics, steroids and immune suppressing drugs contributing factors for mycotic disease. There are 3 categories of risk factors associated with mycosis:1) factors promoting fungal colonization 2) factors suppressing the host immune response and 3) factors that provide a direct route for fungal invasion and infection. Additionally, antibiotic therapy induces a disturbance in udder homeostasis and inhibits neutrophils and T lymphocyte activity, ultimately leading to fungal cell proliferation within the host. Indeed, all cases assessed in this study were fatal cases on IMIs, with susceptibility to antibiotics demonstrated in pathogenic bacterial isolates [6]. Further microbial analysis identified fungal species with clear antifungal resistance present for certain antifungal agents. The etiological agents of mycotic mastitis in this study included Candida albicans, non- albicans Candida (NCAC), Cryptococcus neoformans, Trichosporon lactis and Wickerhamomyces anomalus. Amphotericin B is a fungicidal antifungal drug commonly used for the treatment of Candida mycosis exhibiting effect by binding to cellular ergosterol. Amp B, therefore, induces cell death by creating pores in the fungal cell membrane and the accumulation of reactive oxygen species (ROS) leading to cell damage, apoptosis and death [9]. All isolated species studied demonstrated susceptibility to Amp B with T. lactic the most susceptible species. Trichosporon is a medically important genus associated with gastrointestinal, respiratory tract, skin, and vaginal infections in humans. As an environmental pathogen T. lactic can cause invasive trichosporonosis once entry to the host has been achieved. Amp B binds to ergosterol and to cholesterol (a key component of mammalian cell membranes) with lower affinity, hindering its therapeutic use. Toxicity associated with Amp B includes kidney (renal) cell membrane damage, modulation of intracellular trafficking and eliminating the pH gradient between the endosomes/vacuole and the cytosol [10]. C. albicans, C. tropicalis and T. lactis possessed resistance to fluconazole and low susceptibility to amphotericin B and caspofungin. Indeed, all other isolated species show low levels of susceptibility to fluconazole up to 200µg/ml with zones of inhibition not exceeding 8.7mm. Resistance to azoles is commonly reported in Candida species and is related to alterations in the target enzyme 14α-lanosterol demethylase (14-DM), which is responsible for the production of an ergosterol precursor, encoded by the ERG11 gene. Ergosterol is essential for the fungal cell membrane therefore this inhibition has a fungistatic effect arresting cell growth. Efflux pumps are also associated with Candida resistance due to the CDR1, CDR2 and MDR1 genes [2]. Fluconazole is frequently prescribed for Candida infections in humans resultant from the 5 most common species associated with disease i.e. Candida albicans, Candida glabrata, Candida parapsilosis, Candida tropicalis and Candida krusei [11] all of which are zoonotic in nature. As it emerges that cases of candidemia in animal and humans are frequently resultant from NCAC species it is now essential to establish the level of antifungal resistance in these species. Additionally, Cryptococcus neoformans is an important pathogen frequently associated with pulmonary cryptococcosis and cryptococcal meningitis in immunocompromised persons having impaired cell-mediated immunity. The echinocandin caspofungin proved the most effective antifungal used as the greatest inhibition was evident, except for T. lactic which proved quite resistant, at 25µg/ml. C. tropicalis also appears more sensitive to Amp B than the other antifungal agents. Both important zoonotic species C. neoformans and C. krusei displayed susceptibility to all antifungal agents tested in the following order of decreasing sensitivity: caspofungin, Amp B and lastly fluconazole. A similar pattern emerged for W. anomalus. Echinocandin drugs are the favoured therapeutic for the treatment of candidiasis having effect by inhibiting the biosynthesis of critical glucan polymers in fungal cell walls. Echinocandin resistance in susceptible species, however, is emerging and is typically acquired during therapy [12]. The mechanism of caspofungin resistance is due to amino acid alterations in glucan synthase, decreasing the sensitivity of this enzyme to the antifungal. The echinocandins have the added benefit of an excellent therapeutic index with low mammalian renal and hepatic toxicity or severe drug-drug interactions making them a safer antifungal option. Pasteurisation remains the method of choice for the processing of milk pre-consumption to ensure consumer safety. Previous studies reported by this research group demonstrated the thermal resistance of pathogenic bacterial isolates associated with bovine mastitis [6]. Similarly, HTST pasteurisation of mycotic species demonstrates their resistance to thermal inactivation with all isolated species surviving treatment. T. lactic proved the most resistant with 4.5 log10cfu/ml remaining viable following HTST pasteurisation followed by W. anomalus (4 log10cfu/ml), C. krusei (3.7 log10cfu/ml), C. tropicalis (3.7 log10cfu/ml), C. albicans (3.2 log10cfu/ml), and lastly C. neoformans (2.7 log10cfu/ml). importantly, there was an increase in antifungal resistance seen in C. albicans, C. krusei, C. neoformans and T. lactic post heat treatment indicating that sublethal thermal processing proliferates antifungal resistance to Amp B. This may relate to the presence of heat shock proteins (HSPs) in these species being activated following sublethal exposure. Indeed, studies report the role of HSPs in the proliferation, drug resistance, virulence and biofilm formation of C. albicans [13] and C. neoformans [14]. This is an important find as thermal processing of dairy products at these parameters appears ineffective at protecting the food chain. Invasive fungal infections (IFIs) resultant from contaminated food consumption can cause devastating illnesses and considerable mortality, however, quantifying their public health burden remains challenging [15]. Consequently, the true risk and rate of foodborne IFIs are unidentified. The manifestations and outcomes of foodborne IFIs relate to the fungus and host factors, where immunocompromised persons are particularly at risk. Controlling fungal food spoilage and preventing post consumption pathogenesis is a major issue for food industries where there is an urgent need for efficient solutions to prevent fungal transmission and food contamination in dairy products. Both biocidal options investigated provided excellent antifungal activity with the lowest concentration of each displaying similar activity to the standard IP70%. Additionally, biocidal chemicals peracetic acid and triameen show potential to act as intermediate level disinfectants according to European testing requirements (BSEN 1650). Preventing microbial transmission at all stages of post-harvest, harvest and pre-harvest using effective biocidal solutions is vital to ensure food safety. Effective disinfection of animal housing facilities, milking equipment and milk bulk tanks remains the best prophylactic means of protecting animal health, the food chain and consequently, human health, a key goal of the “One health” initiative.
Conclusion
Antifungal resistance is an ongoing issue, particularly as fungal infections are recognised as a major health problem causing approximately 300 million infections globally with 1.35 million deaths annually. With the increasing awareness that mycotic species are often associated with pathogenesis in both animal and human hosts, there is an immediate need to determine the levels of drug resistance in fungal and yeast zoonotic species. A better understanding of drug resistance including resistance mechanism, cellular and clinical factors promoting resistance and novel methods of overcoming resistance will promote more effective strategies for protecting animal health, the food chain and subsequently, human health. Furthermore, with the environmental impact of climate warming an increase in the incidence of fungal disease is likely to emerge, globally. Findings reported herein detail fungal resistance to Amp B, fluconazole and caspofungin in zoonotic fungal species associated from bovine mastitis where animal culling was conducted to prevent disease transmission. Biocidal options peracetic acid and triameen demonstrate effective antifungal activity in line with the testing requirements of European testing standard (EN1650).
References
- Ksouri S, Djebir S, Hadef Y, Benakhla A (2014) Survey of bovine mycotic mastitis in different mammary gland statuses in two north-eastern regions of algeria. Mycopathologia 179(3-4): 327-331.
- Du J, Wang X, Luo H, Wang Y, Lui X, et al. (2018) Epidemiological investigation of non-albicans Candida species recovered from mycotic mastitis of cows in Yinchuan, Ningxia of China. BMC Veterinary Research 14(1): 251.
- Dworecka KB, Krutkiewicz A, Szopa D, Kleczkowski M, Biegańska M (2012) High prevalence of candida yeast in milk samples from cows suffering from mastitis in poland. The Scientific World Journal 196347: 1-5.
- Becker ATA, Castagnaro D, Bortoli K, Souza C, Drunkler DA, et al. (2016) Mycotoxins in bovine milk and dairy products: a review. Journal of Food Science 81(3): 544-552.
- Lee JH, Kim YG, Gupta VK, Manoharan RK, Lee J (2018) Suppression of fluconazole resistant Candida Albicans biofilm formation and filamentation by methylindole derivatives. Frontiers in Microbiology 9: 2641.
- Meade E, Savage M, Garvey P, Slattery MA, Garvey M (2019) Antibiotic resistant zoonotic pathogens of bovine mastitis and possible agents of foodborne disease. Cohesive Journal of Microbiology and Infectious Disease 2(5).
- Meade E, Garvey M (2018) Efficacy testing of novel chemical disinfectants on clinically relevant microbial pathogens. Am J Infect Control 46(1): 44-49.
- Meade E, Rawe S, Fowley C, Slattery MA, Garvey M (2019) An assessment of alternative therapeutic options for the treatment of prolonged zoonotic fungal infections in companion animals. Journal of Microbial Biotechnology 4(3).
- Mesa AAC, Rueda C, Román E, Quintin J, Terrón MC, et al. (2016) Cell wall changes in amphotericin b-resistant strains from candida tropicalis and relationship with the immune responses elicited by the host. Antimicrobial Agents and Chemotherapy 60(4): 2326-2335.
- Kagan S, Ickowicz D, Shmuel M, Altschuler Y, Sionov E, et al. (2012) Toxicity mechanisms of amphotericin b and its neutralization by conjugation with arabinogalactan. Antimicrobial Agents and Chemotherapy 56(11): 5603-5611.
- Berkow E, Lockhart S (2017) Fluconazole resistance in Candida species: a current perspective. Infection and Drug Resistance 10: 237-245.
- Perlin DS (2015) Mechanisms of echinocandin antifungal drug resistance. Annals of the New York Academy of Sciences 1354(1): 1-11.
- Gong Y, Li T, Yu C, Sun S (2017) Candida albicans heat shock proteins and HSPS-associated signalling pathways as potential antifungal targets. Front Cell Infect Microbiol 7: 520.
- Eastman AJ, He X, Qiu Y, Davis MJ, Vedula P, et al. (2015) Cryptococcal heat shock protein 70 homolog ssa1 contributes to pulmonary expansion of cryptococcus neoformans during the afferent phase of the immune response by promoting macrophage m2 polarization. The Journal of Immunology 194(12): 5999-6010.
- Benedict K, Chiller TM, Mody RK (2016) Invasive fungal infections acquired from contaminated food or nutritional supplements: a review of the literature. Foodborne pathogens and disease 13(7): 343-349.
© 2020 Mark Anthony Slattery. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






