- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Antibiotic Resistant Zoonotic Pathogens of Bovine Mastitis and Possible Agents of Foodborne Disease
Elaine Meade1, Micheal Savage2, Paul Garvey3, Mark Anthony Slattery4 and Mary Garvey1,2, 4*
1 Department of Life Science, Institute of Technology, Ireland
2 Lir Analytical LTD, Ireland
3 Clontygrigney, Ireland
4 Mark Anthony Slattery, Ireland
*Corresponding author:Mary Garvey, Department of Life Science, Institute of Technology Sligo, Ash lane, Sligo, Ireland
Submission: June 12, 2019; Published: July 18, 2019

ISSN 2578-0190 Volume2 Issue5
Abstract
Bovine mastitis is an inflammatory reaction of the udder or mammary gland of the cow, following colonisation with microbial pathogens. Bovine mastitis results in significant economic losses globally, resultant from a reduction in milk yield and quality in addition to treatment costs and animal culling. The disease can exist asymptomatically in its sub-clinical form however, it can quickly manifest into a clinical state where the host, pathogen and environment are all intricately linked. Antimicrobial resistance is increasing among mastitis associated pathogens, in particular those pathogens found in the cow’s environment. Studies were conducted to determine the resistance of mastitis isolates to a range of antibiotic drug classes by use of the Kirby Bauer assay as described by EUCAST. Studies conducted also determine the antimicrobial capacity of four licensed veterinary antibiotics, marbofloxacin, penicillin-streptomycin, trimethoprim-sulfamethoxazole and amoxicillin/clavulanic acid and three marketed teat antibiotic therapy agents on pathogens isolated from chronic cases of mastitis. Additionally, suspension tests were utilised to determine the ability of two biocidal solutions to provide high levels of bacterial cell death as required by European disinfectant requirements. Findings demonstrate activity of all tested products against pathogenic species however, multidrug resistance is evident for a broad range of antibiotic drug classes. Additionally, all strains possess heat resistance with some displaying increased antibiotic resistance post heat stressing. Novel biocide solutions tested for use in veterinary areas provided high levels of microbial inactivation for all test species. Findings suggest that peracetic acid and triameen may be suitable disinfectants for use in veterinary and farm areas, where all multidrug resistance species were susceptible to treatment including spores of B. cereus. Additionally, with the new European residual levels implemented for chlorine and quaternary based products the findings of this study suggest that peracetic aacid and triameen may offer alternative options for use at milk harvest.
Keywords: Mastitis; Multi-drug resistant; Pathogenic; One-Health; Food production; Dairy
Abbreviations: AMR: Antimicrobial Resistance; BSA: Bovine Serum Albumin; CFU: Colony Forming Units; CM: Clinical Mastitis; ESBL: Extended Spectrum Beta Lactamase; EUCAST: European Committee Antibiotic Susceptibility Testing; IMI: Intramammary Infection; LPS: Lipopolysaccharide; MDR: Multi Drug Resistance; PCR: Polymerase Chain Reaction; SCC: Somatic Cell Count; SCM: Sub-Clinical Mastitis; TVC: Total Viable Count; WHO: World Health Organisation; YE: Yeast Extract
Introduction
Mastitis, an inflammatory response of the udder typically to an infectious agent, continues to pose a serious economic threat to the dairy industry. Bovine mastitis in particular causes considerable annual economic losses of up to €16-26 billion globally while also negatively impacting on animal health and wellbeing [1]. Worldwide, mastitis is a challenging endemic disease of diverse aetiology that is directly influenced by the host, pathogen and the environment, the triad of disease. As such, economic losses vary across the globe depending on the animal breed, age, climate and husbandry practices [2] with further expenses owing to the cost of treatment and veterinary consultations. The greatest economic cost of mastitis relates to the poorer quality and reduced volume of milk produced by the infected cow, accounting for a considerable 70% of production losses. This in turn can lead to premature culling and reproductive problems for the animal, where mastitis and calving difficulties have been reported as the top identifiers for bovine morbidity and mortality [3]. Additionally, the disease has a detrimental effect on the animal’s condition which represents non-compliance with the “One Health” system established to protect animal and human welfare. Mastitis appears most often as a subclinical asymptomatic low-grade intramammary infection (IMI) but can manifest into acute clinical forms. Sub clinical mastitis (SCM) typically shows no outward signs of infection in either the animal or milk, making diagnosis difficult, often resulting in the disease persisting for entire lactations (chronic infection) where an infected cow acts as a pathogenic reservoir for healthy cows. On the majority of dairy farms SCM is considered the most economically destructive, where the milk appears normal however, damage to milk producing cells (alveoli) during infection results in an influx of immune somatic cells into the udder reducing milk quality and production. The disease is more common among older lactating cows and can be detected by enumeration of these somatic cells in milk, with high cell levels considerably diminishing both the quality (shelf life) and quantity of milk proteins, specifically casein. In today’s industry, it is imperative that dairy producers maintain low somatic cell counts (SCCs), as exceeding levels are met with price-incurred penalties, with processers applying incentives for milk with lower counts. Furthermore, studies suggest that contamination of milk by somatic cells can lead to allergies in humans such as asthma, colic and childhood diabetes following consumption [4]. Unlike its preceding subclinical form, chronic mastitis (CM) is manifested by physical morphological inflammatory changes in the affected quarter as well as visible abnormalities (clots, flakes) in the milk. The signs and severity of CM vary considerably (mild, moderate, severe), with the degree of illness primarily influenced by parity of the cow and the pathogen involved. Milk production can be profoundly reduced in severe cases with some quarters becoming agalactic leading to culling of the animal. IMIs can be caused by a range of microorganisms, broadly split into two categories;environmental pathogens (Escherichia coli, Streptococcus uberius, Bacillus spp, Pseudomonas aeruginosa) that reside in the cow’s environment (housing, bedding, faeces) and contagious pathogens such as Staphylococcus aureus and Streptococcus agalactiae that originate in the mammary gland, and are easily transmitted from cow to cow (horizontal transmission) particularly during harvest. Up until recently, milk culture was rarely necessary for diagnosis of mastitis, with broad-spectrum antibiotics such as β-lactams (penicillin’s, cephalosporins), tetracyclines and sulphonamides typically prescribed without identification of the causative agent(s). However, the emergence of antimicrobial resistance (AMR) has since directed further complications for veterinarians and farmers, with more and more microbes adapting resistance mechanisms (efflux pumps, alteration/modification of target drug sites, horizontal gene transfer) to overcome drug effects [5]. AMR not only threatens animal health but also poses a serious risk to human health, with the possibility of zoonotic strains entering the food chain.
In 2017 the World Health Organization (WHO) released a list of priority pathogens; present on this list are several mastitis pathogens, namely Escherichia coli (critical), Pseudomonas (critical) and Staphylococcus aureus (high priority). Furthermore, WHO endorsed a list of critically important antimicrobials for human medicine; which includes various compounds for the treatment of mastitis i.e. 3rd & 4th Cephalosporins, fluoroquinolones and penicillin’s [5]. Therefore, there is an immediate need to determine the exact causative agents of mastitis and their level of resistance to both veterinary and human antimicrobial therapeutics. Establishing a resistance profile for these zoonotic pathogens can aid in identifying optimal treatment options for the infected animal and aid in protecting public health safety, a key goal of “One Health”. This study aims to provide such essential information by detailing the resistance profile of numerous zoonotic microbial species isolated from cases of bovine mastitis. Additional studies will determine the susceptibility of isolated species to food perseveration methods (pasteurisation) and novel veterinary disinfectants for use in animal housing and at milk harvest as a means of preventing environmental mastitis transmission and outbreaks. Disinfectants will be non-chlorine and non-quaternary ammonium compounds (QAC) in an effort to limit the use of and subsequent contamination of dairy produce with these toxic chemicals.
Methods
Intramammary infections
Samples from dairy cows showing signs of mastitis were taken and microbial culture performed to isolate species and identify causative agents of disease. Cases of disease included an 11-year-old Holstein/British Friesian cross lactating cow presenting with inflamed udder and mastitis just 2 days post-partum. Farmer observed early signs of udder heat and swelling in cranial teat 1-3 days before physical symptoms, which led to painful, red swollen glands where milk composition was watery with the presence of clots. Initially prescribed IM amoxicillin/clavulanic acid (amox/clav) at a dose of 140/35mg for 3 to 4 days. The animal’s health improved over the next 10 days before clinical symptoms re-occurred. Patient further prescribed Tylosin (10mg per kg per day for 4 days) and marbofloxacin (100mg x 3 days) to which the cow was unresponsive. Animal was culled from the herd. Second case included a 6-year-old Holstein/British Friesian cross lactating cow presenting with similar, but not identical signs of mastitis 3 days post-partum. Clinical signs of heat, swelling, hardness and pain were present in all but one quarter (left-front). Patient unresponsive to amox/clavulanic acid (140/35mg), tylosin and marbofloxacin. Right forefront teat amputated to prevent risk of septicemia. Cow eventually culled from the herd. Third case included a 2-year-old Holstein/British Friesian cross lactating cow presenting with acute clinical signs of mastitis three weeks prior to first-ever calving. Very swollen, heated (to touch), painful clinical signs present in the right forefront quarter. Milk sample was thick and curdled with the presence of blood clots. Patient successfully treated with tylosin 200mg x 4 days, albeit the quarter was lost due to drying up. Microbial analysis identified various pathogenic bacterial Gram-positive and Gram-negative species in all cases of disease with total viable counts (TVC) of 1x107 TVC/mL (case 1), 1.4x105 TVC/mL (case 2) and 3x106 TVC/mL (case 3) present. Pathogenic species isolated from all 3 cases and used for this study are as follows S. uberius, B. cereus, S. aureus, A. buamannii, P. aeruginosa and E. coli.
Microbial isolation and identification
Collected samples of intramammary infection were inoculated in nutrient broth (Cruinn Diagnostics, Dublin, Ireland) and incubated at 37 °C for up to 24 hours before streaking onto nutrient agar (Cruinn Diagnostics, Dublin, Ireland). Bacterial total viable cell counts were performed by diluting milk samples in sterile phosphate buffered saline (PBS) with standard plate counts performed after 24 hours incubation. Individual colonies were re-streaked for isolation and pure isolated colonies inoculated into nutrient broth for further biochemical characterization. Colonies were identified based on their morphological characteristics, biochemical profile and growth on selective agars, specifically CHROMagar™ MRSA, CHROMagar™ Pseudomonas (CHROMagar, Paris, France), Harlequin™ E.coli/Coliform Medium, Baird Parker agar (LabM, Cruinn Diagnostics, Dublin, Ireland), Acinetobacter buamannii selective agar (CHROMagar, Paris, France). Identity was confirmed via colony polymerase chain reaction (PCR). Specifically, single colonies of each bacterial isolate were sub cultured in nutrient broth and incubated overnight at 37 °C. Genomic DNA was directly extracted using the GenElute™ Bacterial Genomic kit (Sigma, Ireland) according to the manufacturer’s instructions and bacterial primers UniF 5’- AGAGTTTGATCCTGGCTCAGG -3’ and UniR 5’- ACGGCAACCTTGTTACGAGT-3’ (Sigma Aldrich, Dublin, Ireland) used for amplification of 16s rRNA gene. PCR was performed in a total reaction volume of 20µl, containing 17µl red Taq 1.1x master mix (VWR, Dublin, Ireland) 1µl UniF, 1µl UniR and 1µl of pure genomic DNA eluate. DNA amplification was performed in a thermo cycler (VWR, Dublin, Ireland) using the recommended parameters. Clean-up and gene sequencing of PCR products was completed by Source Bioscience (Waterford, Ireland). Strains were stored and cultured in nutrient broth/agar at 37 °C and identity confirmed via Gram stain prior to each experimental set up.
Antibiotic resistance profile
Antibiotic resistance profiles were established using selective agars (CHROMagar™ ESBL CHROMagar™ VRE) (Cruinn Diagnostic, Ireland) and a range of antibiotic susceptibility disks (ThermoFisher Scientific, Ireland) according to the European Committee for Antibiotic Susceptibility Testing (EUCAST) recommendations. Specifically, 100ul of ca. 1x106 cfu/ml of a 6-hour bacterial culture was overlaid on to Muller-Hinton agar plates absent of surface moisture (EUCAST, 2019). An antibiotic inoculated disk was placed in the centre of the plate and incubated inverted for 24 hours at 37 °C. Zones of inhibition were measured in millimetre (mm) where the absence of a zone of inhibition denotes resistance in the organism. Inhibited species were then graded as susceptible (S) or as having intermediate (I) susceptibility according to EUCAST. A resistance profile was established in accordance with the WHO priority pathogen list for each species under investigation with critically important E. coli, A. buamannii and P. aeruginosa assessed for resistance to 3rd generation cephalosporins and a carbapenem amongst other drug classes. MRSA which is listed as high importance was assessed for resistance to vancomycin and quinolones amongst other therapeutics (Table 1).
Kirby bauer disk diffusion assay using mastitis drug therapy
The antimicrobial capacity of a number of licensed veterinary antibiotic agents including marbofloxacin (100mg/ml), Co-Trimoxazole (sulfamethoxazole 200mg and trimethoprim 40mg per ml), penicillin-streptomycin (10,000 units penicillin and 10mg streptomycin) and amox/clav acid (140/35mg/ml) were assessed on activity towards mastitis isolates. The Kirby Bauer assay was carried out as per the EUCAST guidelines to determine the sensitivity or resistance of each test isolate to the respective test antibiotics. Specifically, the Kirby Bauer assay was conducted as per Meade et al. [5] with zones of inhibition measured in millimetre (mm) following 24 hours incubation at 37 °C in the absence and presence of bovine serum album (BSA) at 2 and 4%. BSA concentration of 2 and 4% were chosen as the blood concentration of serum albumin in lactating cows with sub-clinical and clinical mastitis is within this range (Singh et al., 2014). Albumin is a protein which can bind drugs in circulating blood reducing the distribution to the area of infection, being the mammary gland in cases of mastitis. Zones of inhibition were also performed for 3 teat antibiotic therapy solutions currently on the market as metaphylactic agents in controlling mastitis in dairy cows. Specifically, the active ingredients in these 3 products were 200/50/10mg amoxicillin/clavulanic acid/prednisolone, 330/100mg lincomycin/neomycin and 300/20mg cefapirin/prednisolone in teat antibiotic therapy solution 1, 2 and 3 respectively.
Pasteurisation
Flash pasteurization or high-temperature short-time (HTST) pasteurization is a commonly used standard heat method in which perishable beverages such as milk is subject to high temperature for a short time in order to kill spoilage microorganisms, to allow for extended unrefrigerated storage and to retain flavour. A suspension of each test strain was prepared as aforementioned adjusting to meet a final cell density of 1x10⁷cfu/ml in sterile PBS. 1ml of this microbial suspension was then transferred to 9ml sterile full fat milk giving a test concentration of ca. 1x106cfu/ml equating to the average TVC obtained for mastitis samples. Controls contained sterile full fat milk only. The resulting suspensions were then heated to 72 °C for 15 secs followed by rapid cooling to 4 °C. Subsequently, the test solution was 10-fold serially diluted and 500µl aseptically spread on nutrient agar plates in triplicate. All plates were inverted and incubated at 37 °C for 24 hours. Surviving colonies were counted and reported as log10cfu/ml compared to an untreated control. Antibacterial resistance was re-assessed after the pasteurization process to determine if heat treatment impacts on the levels of resistance of isolates.
Novel biocidal options
The antibacterial agents investigated in this study are pure biocides used as disinfectants alone or in commercial brands. The concentrations described are the concentration of the active component and include the concentration used following manufactures instructions. Test solutions studied for use in veterinary areas and as farm disinfectants utilise peracetic acid or triameen as active ingredients. Peracetic acid is a powerful oxidant capable of oxidising the outer cell membranes of micro-organisms, acting as a biocidal disinfectant. Triameen is a fatty amine derivative and a highly effective antimicrobial agent. Studies by Meade et al. [6] have demonstrated the antimicrobial potential of both disinfectants on reference strains sourced from the American Type Culture Collection (ATCC) bank. In the present study, the activity of both biocides will be determined against multidrug resistant pathogens and causative agents of mastitis in dairy cows where mortality was the end result in some cases.
Kirby Bauer disk diffusion assay using novel biocidal solutions
The Kirby Bauer assay was carried out to determine the effect of disinfectants on microbial species with the presence and absence of an interfering substance on P. aeruginosa, S. aureus, B. cereus and E. coli isolates to incorporate the most resistant Gram-positive and negative strains isolated species. 1mL of ca. 1x10⁷ microbial cells was added to 9mL BSA to give a working microbial count of 10⁶ cells in solution with 3 g/L or 10g/L BSA and with 10g/L YE as interfering substance. Subsequently, 100μL 10⁶ cells/mL microbial suspension were transferred onto replicate agar plates and spread with a sterile L-shaped spreader (Cruinn Diagnostics) to ensure even disruption across the agar surface. Filter disks (6mm) were immersed in the test biocide solutions at concentrations of 0.01, 0.1 and 1 % (v/v) for 15 seconds and excess solution was allowed to drip off the disk. Subsequently, the disk was placed on the inoculated plate. Plates were then incubated for 24 hours at 37 °C. Zones of inhibition were then measured using a Vernier calliper in millimetres as per Meade et al. [6] for each test chemical and each test organism.
Anti-sporicidal activity using novel biocidal solutions
A quantitative suspension test for the evaluation of sporicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas was used. Bacillus cereus spores were produced using the isolated B. cereus strain as per method of Garvey et al. [7] and stored at -20 °C when not in use. For compliance with this test, test chemicals must achieve a 104 cfu/ml reduction of spores in treatment times of less than 60 minutes in the presence of an interfering substance at 20 °C. Test chemical under study included peracetic acid and triameen at varying concentrations at of 2.5, 5 and 7.5% (v/v). Specifically, 1 ml of a 108 cfu/ml spore suspension was added to 8 ml of the test chemical in the presence of 3.0 g/L bovine serum albumin for low level soiling and 10g/L BSA and 10g/L yeast extract (YE) for high level soiling, and 1ml of water and allowed to react for up to 60 minutes at 20 °C. Following varying treatment time points, the reaction was neutralised using 30g/l polysorbate 80 +3g/l lecithin/l-a-phosphatidylcholine from egg yolk (Sigma, Ireland), with 100µl of neutralised samples spread on nutrient agar plates in triplicate, incubated at 37 °C for 24 hours in order to establish a viable cell count.
Antibacterial activity suspension test BSEN 1656
Suspension tests were conducted as per the methods of the European guidelines for antibacterial testing of chemical disinfectants that form a homogeneous physically stable preparation in hard water for use in veterinary areas. Isolated strains from the above described cases of mastitis were used for this suspension test. Test species were cultured for 8 hours after which cell counts were adjusted to 109 cfu/ml with sterile PBS. Chemical test solutions were prepared as per manufacture instructions for use on site and at a concentration above and below this working concentration giving a range of 0.01, 0.1 and 1% (v/v). Prior to testing all reagents are equilibrated to the test temperature of 10 °C using a water bath. Subsequently 8 ml of the test product was transferred to a sterile container with 1ml of sterile water. Afterward, 1ml of microbial suspension containing 1x109 bacterial cells was added. Additionally, 1ml of interfering substance at 3.0g/L BSA and 10g/L BSA with 10g/L YE (Sigma, Ireland) was added with subsequent incubation for 0 to 15 minutes with mixing in a 10 °C water bath. At set intervals of 5, 10 and 15 minutes 1ml of the test mixture was transferred into a tube containing 8ml neutralizer and 1ml of sterile water. Samples were mixed and incubated in the water bath for 5 minutes. After neutralization 100µl of this bacterial suspension was transferred onto agar plates in triplicate and incubated at 37 °C for 24 hours. Surviving cells of treated organisms was counted to determine the level of bacterial inactivation following exposure to the test solutions compared to the untreated control (PBS). For compliance with this test, test chemicals must achieve a 105 bacterial cell reduction in treatment times less than 30 minutes.
Statistics
All the experiments were performed three times with three plate replicates for each experimental data point providing a mean result for each experimental batch. Average zone diameter was calculated for the Kirby Bauer assay with standard deviation and significance levels at 95% confidence determined for each strain. For suspension testing the log10 reduction was calculated as the log reduction in viable cell numbers (cfu/ml) of the non-treated (N0) and treated (N) samples [log10 (N0/N)]. Student T tests were conducted to determine significance levels (p<0.05) of bacterial susceptibility to treatment and levels of susceptibility or resistance between species investigated using Minitab 16 (Minitab Ltd, Coventry, UK).
Results and Discussion
The present study was conducted to determine the level of sensitivity and thermodynamic nature of pathogens isolated from chronic cases of mastitis, their response to veterinary antibiotic therapy and food perseveration techniques. Chronic clinical cases of mastitis are becoming more prevalent and medically difficult to treat as the causative agents of disease display combination mechanisms to resist conventional antimicrobial therapy. Pathogenic species causing mastitis are reported to have developed increasing resistance mechanisms [1], although the evidence of the efficacy of antimicrobials is limited. Furthermore, these pathogenic species are zoonotic by nature and have the potential to enter the food chain at harvest leading to incidence of infection in humans via the consumption of contaminated dairy food items. Moreover, the environmental Enterobacteriaceae pathogens E. coli and A. buamannii, are gaining notoriety for their high levels of pathogenicity and resistance in cases of mastitis, where the lipopolysaccharide (LPS) component of the outer cell membrane is the primary virulence factor [2]. This LPS endotoxin may result in endotoxin induced shock in the diseased animal and carries a risk to human health as it is heat stable and resistant to pasteurisation techniques. Additionally, initiating antibiotic therapy is often post LPS excretion or induces toxin release following bacterial cell degradation therefore, the treatment of endotoxin-induced shock intravenously with electrolytes, fluids and anti-inflammatory therapeutics is often required with mortality occurring in many animals [7]. Food safety concerns also arise in terms of bacterial toxins produced by both S. aureus and B. cereus which are heat stable and can persist in the food produce causing gastrointestinal disease amongst other morbidities. A. buamannii, P aeruginosa, S. aureus are categorised as part of the ESKAPE pathogen list (jointly with Enterococcus faecium, Klebsiella pneumoniae and Enterobacter species), a group of pathogens responsible for the majority of nosocomial infections and displaying high levels of AMR and multidrug resistance (MDR). The presence of extended-spectrum-beta-lactamases (ESBL) enzymes in microbial plasmids confers resistance to beta lactam antibiotics in many species, which enables resistance to penicillin-based agents, cephalosporins and carbapenems. In addition, studies are documenting ESBL resistance in occurrence with other antimicrobial mechanisms leading to the emergence and proliferation of MDR. Table 1 displays the resistance profile of mastitis isolated species to a range of antibiotics with varying modes of action. The WHO critically important species E. coli, P. aeruginosa and A. buamannii show clear resistance to penicillin, 3rd generation cephalosporins, and macrolides (erythromycin), with intermediate susceptibility to carbapenems, amoxicillin/clavulanic acid, colistin and doxycycline, and susceptibility to the quinolone’s levofloxacin and ciprofloxacin also evident. Gram-positive and highly important MRSA displayed resistance to vancomycin and meropenem, and intermediate susceptibility to streptomycin, with susceptibility to quinolones and macrolides evident. The emergence of methicillin resistant S. aureus with intermediate susceptibility (VISA), or resistance to vancomycin (VRSA) represents a significant threat to public health and safety where VISA an VRSA are currently resulting in hard to treat nosocomial infections globally. B. cereus exhibited resistance to 3rd generation cephalosporins, penicillin and low levels of susceptibility to amoxicillin/clavulanic acid. S. uberius displayed the lowest levels of resistance to all antibiotics compared to other isolated species (Table 1). 3rd and 4th generation cephalosporins are grouped by the WHO into one of the top 3 drug classes of “critically-important antimicrobials” for use in human medicine. This observed resistance contributes further to the belief that the emergence of bacterial resistance is proliferated through the use of veterinary antibiotic therapy. The effect of heat treatment on the levels of resistance was determined by re-assessing the zones of inhibition on strains surviving the pasteurisation procedure. Figure 1 displays the survival of test organisms to flash pasteurisation where greater than 3 log10 of all test species remained viable post treatment.The most susceptible species to heat treatment was S. aureus where a ca. 2.8 log10 reduction in viable cell numbers was achieved. European regulations set a maximum cfu/ml of 50,000 viable bacterial cells in milk 5 days after storage at 6oC with a <5 cfu/ml allowable for coliform species.Mastitis strains such as S. aureus have been detected in pasteurised milk samples, Dai et al. [8] reports up to 56.6% of the pasteurized milk samples were positive for S. aureus with Staphylococcal enterotoxins responsible for most food outbreaks [8]. E. coli showed high levels of resistance to pasteurisation with greater than 4 log10 cfu/ml viable after treatment, far exceeding the limits set out by EU regulations.
Figure 1:Log10 cfu/ml of viable and non-viable mastitis isolates following flash pasteurisation of 1x106 cfu/ml (± Standard deviation). A, B, C, D, E, F and G donates significance difference at P<0.05.
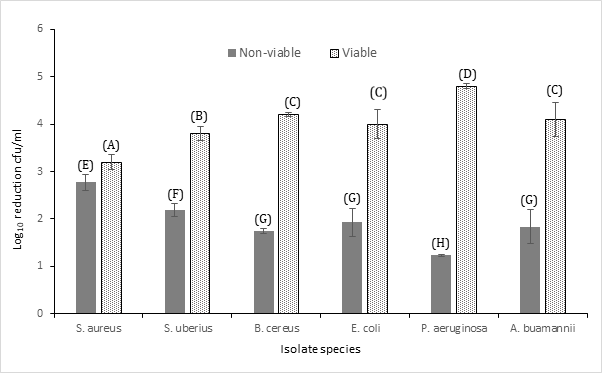
Table 1:Antibiotic profile of mastitis isolates to a range of antibiotics from varying drug classes with EUCAST target zones given for species for which such information is available. Increases in resistance following heat treatment is provided where such increased occurred.

Furthermore, the levels of resistance displayed by E. coli to the following antibiotics significantly increased post heat treatment: chloramphenicol, meropenem, levofloxacin, ciprofloxacin and colistin.This alteration was also evident in the other WHO critically important species A. buamannii and P. aeruginosa (Table 1). Specifically, heat treated A. buamannii showed increased resistance to amoxicillin/clavulanic acid, doripenem, meropenem, ciprofloxacin and colistin with P. aeruginosa displaying increased resistance to chloramphenicol, amoxicillin/clavulanic acid, meropenem, levofloxacin and ciprofloxacin. This was also evident for all Gram-positive species exposed to cefotaxime, doripenem, meropenem, levofloxacin, ciprofloxacin and erythromycin. Possibly heat shock proteins (HSPs) produced by bacterial species exposed to the heated high stress environment induced this elevated level of resistance. HSPs have been associated with antibiotic resistance to beta lactams amongst other drug types [9]. These findings indicate that the process of pasteurisation at the parameters described herein, serve to proliferate resistance in certain already resistant species, an important find in terms of food security and public health safety. The ability of B. cereus to survive heating is attributable to endospore formation jointly with its thermoduric nature. The presence of Bacillus spores in dairy produce particularly infant milk powder represents an ongoing challenge to the dairy industry. Saket et al. [10] reports a prevalence for B. cereus spores in 21% of milk based infant products with the pathogenic Bacillus subtills also present [10], an alarming trend for high risk age groups.
Table 2 depicts the activity of licensed veterinary antibiotics regularly used for the treatment of mastitis. All 4 therapeutics or therapeutic combinations proved efficient at providing high levels of bacterial inhibition. In terms of susceptibility, Gram-negative species (E. coli, P. aeruginosa and A. buamannii) and the Gram-positive B. cereus display high levels of susceptibility to marbofloxacin, C-Trimoxazole (C-T), amox/clav and lastly pen/strep, in decreasing order. Gram-positive species S. aureus showed greater susceptibility to C-T (51mm) followed by marbofloxacin (47mm), amox/clav (42mm) and lastly pen/strep (39mm). S. uberius was more susceptible to marbofloxacin (46mm), pen/strep (42mm) followed by amox/clav and C-T (36mm). Amoxicillin with clavulanic acid consistently proved the least effective antibiotic under study. For all therapeutics under study the presence of BSA at 2 and 4% did influence the diameter of the zone of inhibition. For marbofloxacin there was an increase in activity for E. coli, P. aeruginosa and B. cereus and a significant (p<0.05) decrease for the remaining species investigated (Table 2). This was not uniform for all drugs under study however, with BSA reducing the activity of C-T on all organisms except E. coli and P. aeruginosa. Pen/strep and amox/clav had increased activity in the presence of BSA for P. aeruginosa and A. buamannii. Indeed, BSA provided increased activity of all antibiotics against P. aeruginosa suggesting that the presence of serum albumin somehow increased the cellular uptake of antibiotics by this species. The binding of therapeutics to plasma proteins such as albumin is known to affect the bioavailability, elimination, distribution and half-life of active agents. Protein binding reduces the amount of drug available for bactericidal or bacteriostatic action as bound protein is not pharmacologically active. The effect of protein binding on antibiotics is well documented for β-lactams however, data on other classes of antimicrobial agents is lacking [11]. The findings of this in vitro study suggest that the activity of protein binding may be drug and species specific. Marbofloxacin is a synthetic fluoroquinolone antibiotic that prohibits bacteria growth certified by the FDA for infections in soft tissues and is well established for veterinary use. The particular biochemical interaction of marbofloxacin results in the destruction of bacteria through inhibition of DNA-gyrase and topoisomerase IV. Bactericidal activity on Gram-negative bacteria is influenced by time while bactericidal activity on Gram-positive bacteria is influenced by concentration [12]. This antibiotic is typically administered via intramuscular injection (IM) in a 10% aqueous solution. Studies show a marbofloxacin concentration of 0.5μg/mL was maintained for 5 to 6 hours in the milk of dairy cows given the therapeutic via IM injection for the treatment of coliform (E. coli) mastitis [13]. While a residual level of antibiotic therapy in the milk post treatment offers the benefits of preventing bacterial re-growth particularly at minimum inhibitory concentrations (MICs) there is concern relating to the presence of such residuals in milk which may be used for food production. The unquantified consumption of antibiotic residuals can have untold impact on gut microbiota causing dysbiosis amongst other morbidities in humans. Risk assessment carried out on residues of b-lactams (penicillin, amoxicillin) and macrolides in food products has identified their immuno-allergic potential [14]. Exposure to sub toxic levels of antibiotics also serves to promote resistance in species due to activation of resistance mechanism such as efflux pumps. C-Trimoxazole is an antimicrobial drug originating from potentiation of sulfamethoxazole and trimethoprim. Potentiation decreases the number of sulphonamides necessary to inhibit particular bacteria aiming to decrease animal toxicity and microbial resistance. C-Trimoxazole is extensively used to treat both bacteria and protozoan infections in animals and humans [15] including soft tissue infections like mastitis.
Table 3:Zones of inhibition (mm) produced by test teat antibiotic therapy’s containing 1), 200/50/10 mg amoxicillin/ clavulanic acid/prednisolone, 2) 330/100mg lincomycin/neomycin and 3) 300/20mg Cefapirin/prednisolone on a range of clinical mastitis isolates in the absence (0) and presence of interfering substance BSA at 2 and 4 % (± standard deviation).

Trimethoprim is also a widely used empirical drug for treatment of urinary tract infections (UTIs) in humans. The main route of administration for this medication is via IM injection where a milk withdrawal period of 56 hours is required subsequent to administration. P. aeruginosa and S. uberius displayed the lowest level of susceptibility to this drug in this study with S. aureus being the most susceptible. The findings of this study indicate that marbofloxacin and C-Trimoxazole are more effective options for both environmental and contagious pathogens. In vitro studies however, do not guarantee in vivo activity where additional factors such as the presence of high fat and protein content milk, host immunity and routes of administration also contribute to drug efficacy. Additionally, it is unknown if systemic antimicrobial treatment eradicates the infection through clearance of IMI or via treatment of the bacteraemia that is often present in clinical mastitis cases. Certainly, the treatment of mastitis should be targeting causative species of infection or in sub-clinical cases herd prevalence data should inform therapeutic choices. Preventative measures however, remain the best option in controlling the spread of pathogens at farm level and reducing the usage and risks associated with antibiotic therapy (namely resistance and antibiotic residuals present in dairy food).
Table 2 depicts the activity of licensed veterinary antibiotics regularly used for the treatment of mastitis. All 4 therapeutics or therapeutic combinations proved efficient at providing high levels of bacterial inhibition. In terms of susceptibility, Gram-negative species (E. coli, P. aeruginosa and A. buamannii) and the Gram-positive B. cereus display high levels of susceptibility to marbofloxacin, C-Trimoxazole (C-T), amox/clav and lastly pen/strep, in decreasing order. Gram-positive species S. aureus showed greater susceptibility to C-T (51mm) followed by marbofloxacin (47mm), amox/clav (42mm) and lastly pen/strep (39mm). S. uberius was more susceptible to marbofloxacin (46mm), pen/strep (42mm) followed by amox/clav and C-T (36mm). Amoxicillin with clavulanic acid consistently proved the least effective antibiotic under study. For all therapeutics under study the presence of BSA at 2 and 4 % did influence the diameter of the zone of inhibition. For marbofloxacin there was an increase in activity for E. coli, P. aeruginosa and B. cereus and a significant (p<0.05) decrease for the remaining species investigated (Table 2 &3). This was not uniform for all drugs under study however, with BSA reducing the activity of C-T on all organisms except E. coli and P. aeruginosa. Pen/strep and amox/clav had increased activity in the presence of BSA for P. aeruginosa and A. buamannii. Indeed, BSA provided increased activity of all antibiotics against P. aeruginosa suggesting that the presence of serum albumin somehow increased the cellular uptake of antibiotics by this species. The binding of therapeutics to plasma proteins such as albumin is known to affect the bioavailability, elimination, distribution and half-life of active agents. Protein binding reduces the amount of drug available for bactericidal or bacteriostatic action as bound protein is not pharmacologically active. The effect of protein binding on antibiotics is well documented for β-lactams however, data on other classes of antimicrobial agents is lacking [11]. The findings of this in vitro study suggest that the activity of protein binding may be drug and species specific. Marbofloxacin is a synthetic fluoroquinolone antibiotic that prohibits bacteria growth certified by the FDA for infections in soft tissues and is well established for veterinary use. The particular biochemical interaction of marbofloxacin results in the destruction of bacteria through inhibition of DNA-gyrase and topoisomerase IV. Bactericidal activity on Gram-negative bacteria is influenced by time while bactericidal activity on Gram-positive bacteria is influenced by concentration [13]. This antibiotic is typically administered via intramuscular injection (IM) in a 10% aqueous solution. Studies show a marbofloxacin concentration of 0.5μg/mL was maintained for 5 to 6 hours in the milk of dairy cows given the therapeutic via IM injection for the treatment of coliform (E. coli) mastitis [13]. While a residual level of antibiotic therapy in the milk post treatment offers the benefits of preventing bacterial re-growth particularly at minimum inhibitory concentrations (MICs) there is concern relating to the presence of such residuals in milk which may be used for food production. The unquantified consumption of antibiotic residuals can have untold impact on gut microbiota causing dysbiosis amongst other morbidities in humans. Risk assessment carried out on residues of b-lactams (penicillin, amoxicillin) and macrolides in food products has identified their immuno-allergic potential [14]. Exposure to sub toxic levels of antibiotics also serves to promote resistance in species due to activation of resistance mechanism such as efflux pumps. C-Trimoxazole is an antimicrobial drug originating from potentiation of sulfamethoxazole and trimethoprim. Potentiation decreases the number of sulphonamides necessary to inhibit particular bacteria aiming to decrease animal toxicity and microbial resistance. C-Trimoxazole is extensively used to treat both bacteria and protozoan infections in animals and humans [15] including soft tissue infections like mastitis. Trimethoprim is also a widely used empirical drug for treatment of urinary tract infections (UTIs) in humans. The main route of administration for this medication is via IM injection where a milk withdrawal period of 56 hours is required subsequent to administration. P. aeruginosa and S. uberius displayed the lowest level of susceptibility to this drug in this study with S. aureus being the most susceptible. The findings of this study indicate that marbofloxacin and C-Trimoxazole are more effective options for both environmental and contagious pathogens. In vitro studies however, do not guarantee in vivo activity where additional factors such as the presence of high fat and protein content milk, host immunity and routes of administration also contribute to drug efficacy. Additionally, it is unknown if systemic antimicrobial treatment eradicates the infection through clearance of IMI or via treatment of the bacteraemia that is often present in clinical mastitis cases. Certainly, the treatment of mastitis should be targeting causative species of infection or in sub-clinical cases herd prevalence data should inform therapeutic choices. Preventative measures however, remain the best option in controlling the spread of pathogens at farm level and reducing the usage and risks associated with antibiotic therapy (namely resistance and antibiotic residuals present in dairy food).
Figure 2:Zones of inhibition (mm) for MDR mastitis isolates against (a) triameen and (b) peracetic acid at varying concentrations in the absence (0g/L) BSA and presence of low level interfering agents (3g/L BSA) and high level interfering agents (10g/L BSA and YE)(± S.D.). A, B, C, D, E, F, G, H, I, J, K, L, M, N, P, Q, R, S and T represent siginficant difference at p<0.05.
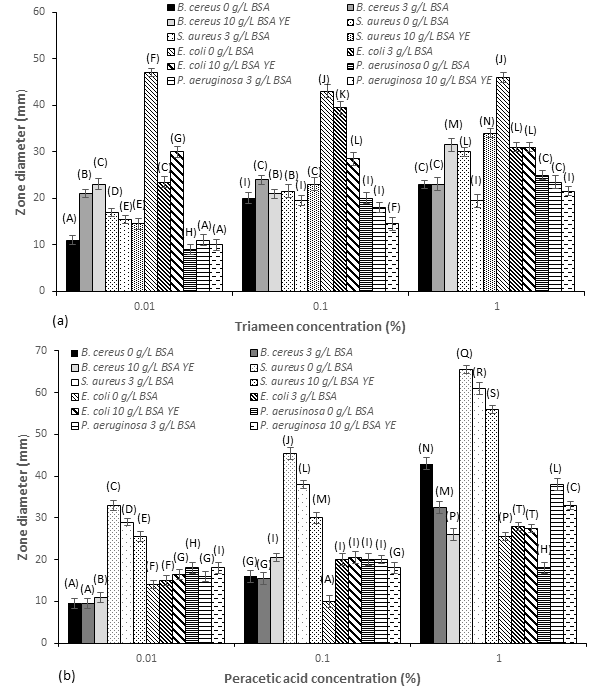
Adequate and efficient use of biocide solutions on farms and in milking management can help prevent incidence of mastitis within herds consequently reducing the need for antibiotic therapy. Figure 2 depicts the zones of inhibition of test solutions of peracetic acid and triameen on isolated bacterial species. All strains proved susceptible to both biocides to varying degrees. E. coli proved most susceptible to triameen (46mm) with activity significantly inhibited in the presence of interfering substance at all concentrations tested (Figure 2a). For triameen at 0.01% no pattern was evident where Gram-positive or negative strains appeared more or less resistant, the order of sensitivity was as follows from most to least sensitive: E. coli, S. aureus, B. cereus and P. aeruginosa. At a concentration of 1% however, there was less variance between strains with B. cereus, S. aureus and P. aeruginosa showing comparable levels of sensitivity. A pattern did emerge however, where Gram-positive species (S. aureus, B. cereus) proved more sensitive to peracetic acid (Figure 2b) than Gram-negative species. Additionally, Gram-negative species appear more sensitive to triameen, where 1% gave a zone of 46mm for triameen and 25.5mm for peracetic acid respectively on E. coli.
Figure 3:Zones of inhibition (mm) for MDR mastitis isolates against (a) triameen and (b) peracetic acid at varying concentrations in the absence (0g/L) BSA and presence of low level interfering agents (3g/L BSA) and high level interfering agents (10g/L BSA and YE)(± S.D.). A, B, C, D, E, F, G, H, I, J, K, L, M, N, P, Q, R, S and T represent siginficant difference at p<0.05.
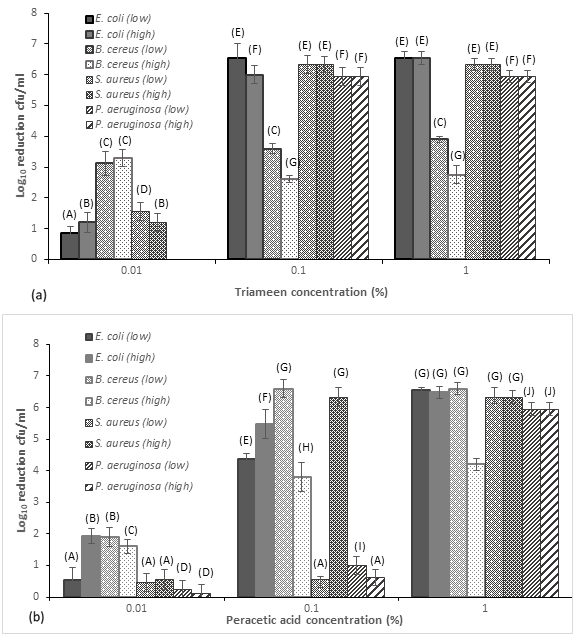
Whereas, a zone of 25mm and 18mm was evident for P. aeruginosa for 1% of triameen and peracetic acid respectively. As with triameen increasing concentrations of interfering substance did reduce the zone of inhibition for peracetic acid suggesting the presence of organic matter influences the activity of the biocide. The effectiveness of biocide chemicals on environemntal and milking machine disinfection is therfore dependent on the level of organic material/interferring substance present. The order of sensitivity to peracetic acid from most to least is as follows: S. aureus, B. cereus, P. aeruginosa and E. coli at 1%. Peracetic acid also provided a 5log10 reduction of B. cereus spores within 45 minutes at concentration of 2.5 and 5%, where triameen failed to achieve similar levels up to 7.5% concentration (data not shown). The sporicidal activity of peracetic acid at this concentration has been reported by Meade et al. [6] for Bacillus strains. Findings of this study agree with the findings of Meade et al. [6] where triameen provided maximal 2 log10 reduction in viabile B. cereus spores. Peracetic acid provided a 5 log10 reduction within 45 minutes meeting the requiremetns of BSEN quantitative suspension test for the evaluation of sporicidal activity of chemical disinfectants. Figure 3 depicts the suspension test for disinfectants used in veterinary areas as per the methods of BSEN 1656 at 15 minutes treatment time. For triameen 0.1% provided the necessary 5 log10 inactivation of E. coli, S. aurues and P. aeruginosa (Figure 3a) as required by BSEN 1656 in both low and high level soiling. B. cereus however was more resistant to treatment where a maximal 3.6 log10 cfu/ml inactivtion was achieved in low level soiling at a concentration of 0.1%. No significant increase in inactivation was ahcieved when increasing the traimeen concentration to 1% making 0.1% a suitable concentration for disinfectation procedures. As with the Kirby Bauer assay no pattern emerged where Gram-Negative or Gram-positive species appear more or less sensitive to triameen induced cell death. Peracetic acid produced a 5 log10 reduction of all test strains with E. coli proving most sensitive to biocidal application. A concentration of 1% was required however to meet the requirements of BSEN 1656 in 15 minutes for P. aeruginosa where 0.1% was sufficient for E. coli, B. cereus and S. aureus for peracetic acid. Findings demonstrate that both test chemicals have good potential for the non-antimicrobial control of bacterial IMI pathogens of contagious and environemental origin. With the issues of QAC, chlorine and iodine residues present in dairy food items, a wide range of alternative disinfectant options have become available containing different active ingredients. To date however, there is limited information available on their ability to inhibit bacterial growth [16]. The findings of this study report the efficacy of two disinfection options, namely peracetic acid and triameen based solutions, to reduce the viability of a range of MDR mastitis isolates. In developed countries the methods of dairy farming have moved towards automated systems reliant on milking machines or alley scrapers which means that optimal machine disinfection is key in controlling pathogen transmission. Adequate machine and housing disinfection is both labour and knowledge–intensive however, with its own challenges such as chemical residual levels in the bulk milk tank and milk itself. Futhermore, in developed countries there has been a major decrease in the prevalence of contagious mastitis and a relative increase in the incidence of environmental mastitis Klaas I, Zadoks R [2] suggesting that housing and farm disinfection practices may help to control enviromental species.
Evidence Based Veterinary Medicine (EBVM) and decision making is becoming increasingly important for veterinarians and farmers in the control of IMIs, promoting udder and animal health and farm economics. The use of non-antimicrobials in reducing IMIs at herd and cow level in lactating and dry cows will play a key role not only in ensuring animals remain disease free but also in curbing the emergence of AMR and pollution of food items with antibiotics. Coupled with the need for new improved tools for mastitis management and control there is also a need to communiate and inform all stakeholders involved in dairy production. Sourcing available and appropriate evidence for use in treatment decision making is the foundation of evidence based medicine. EBVM contributes to better clinical decisions, enhancing mastitis control and directing future mastitis research. The data presented herein, provides in vitro evidence demonstrating the efficacy of antibiotic, teat antibiotic therapy and biocidal options for use in the control and treatment of mastitis pathogens posing a risk to public health safety [17,18].
Conclusion
Effective IMI preventation and treatment remains essential in any mastitis plan to prevent the transmissoin of pathogens within the dairy herd. As mastiits is a intricate disease with the cow, environment and pathogen all important factors in disease outbreak, the process of bacteriological elimination and resolution of clinical symptoms is multifaceted. Although substantial progress has been made in controlling contagious IMIs, mastitis continues to be the most frequent and economically costly disease of dairy cows. Environmental pathogens tend to be less adapted to survival in the udder and infection often triggers an immunological and systemic response causing clinical symptoms in the animal. The ability of pathogens to colonise the udder and cause infection is associated with the degree of host adaptation to the pathogen. While the therapeutic agents investigated show good activity against isolated strains, the presence of antibiotic residuals in milk remains a serious concern. Futhermore, as mastitis pathogens are consistently acquiring and sharing antibiotic resistance mechanisms, it is increasingly important to prevent disease occurance, therefore, negating the need for drug therapy. Findings show that the transmission of pathogens can be controlled by use of adequate disinfectants in the farm and milking environment and by the use of tools which prevent entry into the teat canal or threat teat infections such as teat antibiotic therapy solutions. Additionally, with the new European residual levels implemented for chlorine and quaternary based products the findings of this study suggest that peracetic acid and triameen may offer alternative options for use at milk harvest.
Conflict of Interest
The authors declare there is no conflict of interest.
References
3. Rollin E, Dhuyvetter K, Overton M (2015) The cost of clinical mastitis in the first 30 days of lactation: An economic modelling tool. Prev Vet Med 122(3): 257-264. Meade E, Slattery MA, Garvey M (2017a) Antimicrobial resistance: An agent in zoonotic disease and increased morbidity. Journal of Clinical and Experimental Toxicology 1(1): 30-37.
© 2019 Mary Garvey. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






