- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Types of Microorganisms at Nosocomial Infections on the Surface of Laryngoscope Before Intubation at General Anesthesia
Asghar Karbord*1, Hamid Kayalha2, Fariba Hashemi3, Mohammad Qasem Roushanfekr4 and Mohammad Bokharaei5 and Maryam Rajabi6
1MSc epidemiology of Medical Microbiology Research Center, faculty members of surgical technologist group of paramedical college, Qazvin University of medical science, Qazvin, Iran
2Professor assistant of anesthesiology, Faculty member of Medicine College of Qazvin University of Medical Sciences, Qazvin, Iran
3MSc educational nursing, faculty members of surgical technologist group of paramedical college, Qazvin University of medical science, Qazvin, Iran
4,6Professor assistant of anesthesiology, Faculty member of Medicine College of Qazvin University of Medical Sciences, Qazvin, Iran
5MSc ICU educational nursing, faculty members of surgical technologist group of paramedical college, Qazvin University of medical science, Qazvin, Iran
*Corresponding author: Asghar Karbord, MSc epidemiology of Medical Microbiology Research Center, Iran
Submission: December 19, 2019; Published: January 06, 2020

ISSN 2578-0190 Volume3 issues3
Abstract
Introduction: Laryngoscope is using for endotracheal intubations. Inadequate decontamination of laryngoscope can develop nosocomial infections. If the blade isn’t disconnecting from laryngoscope after intubation can be transmit infections to handle. This study considering prevalence and types of bacteria isolated separately from laryngoscope blades and handles.
Method: This cross-sectional study was in the operating rooms of the educational and treatment center of Qazvin University of Medical Science in province Qazvin in Iran at 2018. 40 Laryngoscope blades and 40 laryngoscope handles were sampled after disinfection, with 4 methods disinfection: (1-Water, Povidon Iodine 7.5%, Ethanol 70%), (2-Water, Povidon Iodine 7.5%, Deconex (guaifenesin and phenylephrine 53 plus), (3-Water, Povidon Iodine 7.5%) and (4-Ethanol 70%)”. Samples were cultured on Mueller Hinton 5% sheep blood agar plate, MacConkey agar and Manitol salt agar. Inoculating plates were incubated at 37 ᵒC for 48 hours. Dominant microorganism and other growth bacteria identified comparatively.
Results: Nine various types of microorganisms were isolated and determined that handles were more Contamination than blades. Most negative results derived from blades and handles that were separated from each other and also for blades that were kept on gauze and separated from other instruments.
Conclusion: Performance of a standard disinfection method by software spss22 for both part of laryngoscope (blade and handle) seems to be necessary. Considering a special spot (like a special dish) for laryngoscope separated from other instruments may prevent the development of nosocomial infection
Keywords: Laryngoscope; Nosocomial infection; Intubation
Introduction
One of the most important Anesthesia instruments is laryngoscope. Laryngoscope is a type of endoscope used to endotracheal intubation and composed of a blade and a handle [1]. Laryngoscope blade comes in direct contact with the patient mucous membranes or broken skin and can be contaminated with secretions from the mouth and oropharynx, blood and various species of microorganism. Laryngoscope handle does not usually come in direct contact with patient’s oral mucosa. It can be contaminated indirectly while its routine disinfection is not observed as a standard practice. Laryngoscope blade and handle can transmit a lot of microorganisms to each other when the blade is not removed from handle after use.
When the hand of anesthesia provider touches a contaminated laryngoscope that seems to be clean, microorganisms can transmit to him and to other patients [2]. Also lack of a special keeping spot (for example a special dish) separated from other instruments can cause cross contamination between laryngoscope and other instruments and can spreads nosocomial infections [3-6]. Over two million patients per year develop nosocomial infections, resulting in 90,000 deaths annually and significant added healthcare costs, as well as unanticipated burdens on patients and their families [3]. Laryngoscope blades are placed in the semi critical group of medical instruments classified by Center for Disease Control and Prevention (CDC) and the Association for Professionals in Infection Control and Epidemiology (APIC) and designated by the CDC as “high level” disinfection [5]. In this study the researchers consider the incidence and the type of bacteria isolated from laryngoscope blades and handles which are separately used in an operating room and consider relationship between its contamination and its keeping spot.
Method
This cross-sectional study performed, and Samples were collected from operating rooms laryngoscopes of an educational and treatment center related to Qazvin University of Medical Science in Qazvin in Iran. Out of 80 samples which were earned (random simple sampling) in 4 consecutive days from 9 am to 3pm, 40 samples were taken from laryngoscope blades while other 40 samples were taken from laryngoscope handle. Some information about the laryngoscope that is ready to use include disinfection method, keeping spot, separating blade and handle from each other, and about the patient who was going to be intubated with that laryngoscope. The researchers had no interference in disinfection methods, and it was done within their routine procedures. There were 4 manual disinfection methods: 1- water, Povidon Iodine 7.5%, Ethanol 70% 2-Water, Povidon Iodine 7.5%, Deconex 53 plus 3- Water, Povidon Iodine 7.5% 4-Ethanol 70%. The investigator obtaining the sample wore sterile gloves and held the laryngoscope by its handle to avoid contacting with blade and sampled handle and blade with two sterile swabs separately. Sampling was done in aseptic circumstance. The sterile swab was rubbed to entire surfaces rotatory. After sampling, both divisions of laryngoscope were cleaned with ethanol 70% and prepared for next use. Nutrition broths (Merk) including samples transported to a laboratory with an ice box with a maximum transporting time of 4h. Specimens were inoculated onto a Mueller Hinton 5% sheep blood agar plate (High Media), MacConkey agar and Manitol salt agar (High Media). Inoculated plates were incubated at 37 ᵒC with 5%-10% CO2 for 48 hours. The result of Bacterial culture was accomplished using standard laboratory methods and dominant microorganism and other growth bacteria identified comparatively. Also, number of colonies was reported approximately. Data was entered in Statistical Package for Social Sciences (SPSS version 22) and all the data was analyzed. Data for baseline characteristics type of microorganisms was expressed as statistical relationship by Chi-Square test. In our study all the data was collected on medical checkups. Analysis of the data was done using descriptive statistics like frequencies, percent of microorganisms.
Results
From 40 samples taken from laryngoscope blades 28(70%) were positive and from 40 samples taken from laryngoscope handles 33(82.5%) were positive. Nine different microorganisms were isolated: 1-Staphylococci, 2- Streptococci spp, 3-Bacillus spp, 4-Neisseria spp, 5-Enterobacter spp, 6-Diphtheria spp, and 7- Strep- Grp-A, 8-Yeast, 9- B-hemolytic streptococci. (Table 1) Dominant Organism in blade samples 10(25%) was Gram-Positive Bacillus and in handle samples 18(45%) was Gram-Positive Cocci. From all 80 samples 11(13.7%) staphylococci Coagulase-positive were found that 4(36.3) were for blades and 7(63.6) were for handles. From 40 collected blade samples most, negative cases were related to those were put on gauze separate from other instruments. From 80 samples 60(75%) blade and handle were separated that 19(31.6%) cases were negative and 20(25%) blade and handle were not separated that 1(20%) were negative. From 4 disinfection methods most, negative cases resulted in complex of water, povidon- iodine 7.5% and deconex (Table 2).
Table 1: Frequency of various bacteria from laryngoscope blades.
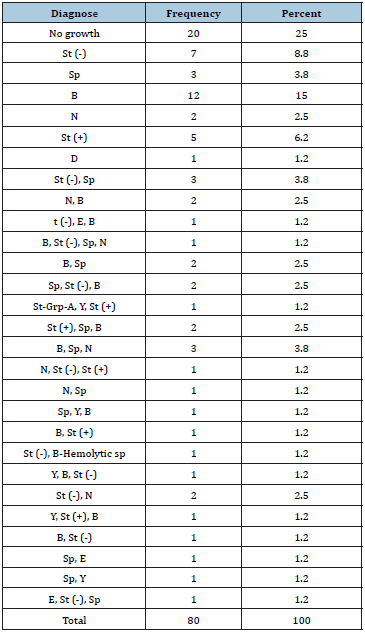
St= Staphylococci; Sp= Streptococci spp; B= Bacillus spp; N= Neisseria spp; D= Diphtheria spp; E= Enterobacter spp; Y= Yeast spp; (+) =Coagula’s Positive; (-) =Coagulase Negative
Table 2: Frequency of various bacteria from laryngoscope blades by methods disinfection.

St= Staphylococci; Sp= Streptococci spp; B= Bacillus spp; N= Neisseria spp; D= Diphtheria spp; E= Enterobacter spp; Y= Yeast spp; (+) =Coagula’s Positive; (-) =Coagulase Negative
Discussion
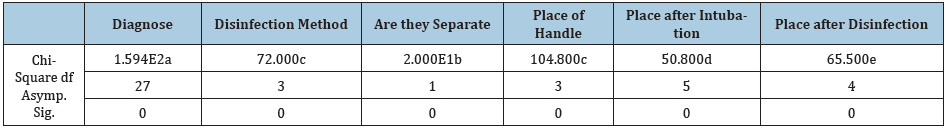
In this study the researcher’s different microorganisms on apparently clean laryngoscope that some of them are pathogen and potential for nosocomial infections which indicate that the decontamination procedures are disappointing and are not adopted to international standards. Also, the researchers found a significant relation between disinfection method and contamination quantity (p≤0.05 was significant). And there is a significant relation between keeping blade and handle separated from each other (p≤0.05 was significant) (Table 3). Also, more positive samples on handle laryngoscope indicate inadequate attention to handle disinfection that may cause contamination transmission to clean blade and to another instrument. In 2009 a study in the State University of New York Upstate IRB (Syracuise NY) 60 laryngoscope handles were sampled after low-level disinfection. From 40 samples sent for aerobic bacterial culture 30(75%) was positive and from 20 samples sent for viral testing all was negative [2]. In 2007 an outbreak of Pseudomonas aeruginosa in a neonatal intensive care unit (NICU) was identified that two infants reportedly died. Improper reprocessing of rigid laryngoscopes was identified as the cause of this outbreak [4]. This study supports previous studies and emphasizes laryngoscope disinfection adopted with standard methods. We also suggest considering a special spot or dish for laryngoscope separated from other instruments.
Table 3: Significant relation between disinfection method and place of laryngoscope.
Conclusion
Inadequate attention to decontamination of laryngoscope causes a lot of pathogen microorganism to be remained on this instrument. Teaching of an effective method for decontamination of laryngoscope designed by Center for Disease Control and Prevention (CDC) and supervising its performance in a best way seems to be necessary. Also considering a special spot or special dish for laryngoscope separated from other instruments may prevent the development of nosocomial infection [7].
References
- Simmons SA (2000) Laryngoscope handles: A potential for infection. AANA 68(3): 233-236.
- Tyler RC, Frederic JA, Scott WR, Deanna LK, Sumena C, et al. (2009) Nasocomial contamination of laryngoscope handles: challenging current guidelines. Anesth Analg 109(2): 479-483.
- Laurie A, Roming, David Hudak, Jeff Barnard (2005) Scrub making the case for disposable laryngoscope blades. Emerg Med Serv 34(3): 91-94.
- Muscarella LF (2008) Reassessment of the risk of healthcare-acquired infection during rigid laryngoscopy. Journal of Hospital Infection 68(2): 101-107.
- Ying HC, Karlokwong, Japing S, Yin ching C, Yichueh, et al. (2006) Use of condoms as blade covers during laryngoscopy, a method to reduce possible cross infection among patients. J Infect 52(2) 118-123.
- MJL Bucx, Dankert J, Beenhakker MM, Harrison TEJ (2001) Decontamination of laryngoscopes in the Netherlands. Br J Anaesth 86(1): 99-102.
- Junichi O, Kouichiro M, Hiroshi M, Takafumi H, Midori O, et al. (2004) Gargling with povidone-iodine reduces the transport of bacteria during oral intubation. Can J Anaesth 51(9): 932-936.
© 2020 Asghar Karbord. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






