- Submissions

Full Text
Biodiversity Online J
The Tolerance of Citrus Seeds to Desiccation Reveals a Range of Behaviors from Orthodox to Recalcitrant
François Luro*, Bruno Tomu and Isabelle Tur
UMR AGAP Institute, University Montpellier, France
*Corresponding author:François Luro, UMR AGAP Institute, University Montpellier, CIRAD, INRAE, Station INRAE 20230 San Giuliano, France
Submission: September 23, 2025; Published: October 08 2025

ISSN 2637-7082Volume5 Issue 4
Abstract
The conservation of citrus seeds is limited in time because they lose their germination capacity after few months at 20 °C. A broad evaluation of the seed desiccation tolerance was carried for 41 varieties from the main species of Citrus, Fortunella and Poncirus genus. Seed Water Content (WC) and fruit size are relevant indicators for assessing the optimal maturity of seeds prior to processing. Seed water content and temperature are key factors in sensitivity to desiccation. After 30 days at 25 °C, seeds undergo accelerated ageing at varying rates depending on the species. Water exchange between seeds and the atmosphere varies depending on the genus. It is lower in Poncirus trifoliata, leading to higher seed moisture content than in varieties of the Citrus species. Trifoliate oranges and kumquats are very sensitive to desiccation and the WC50 threshold (50% of germination loss) is high (around 28%), and their germination is null at 20% of WC. Citrons (C. medica) and their relatives, limes and lemons, are highly tolerant to low WC (<6%) and so they seed could be considered as orthodox. Grapefruit and pummelo seeds are medium tolerant while mandarin and sweet orange seeds are sensitive to 12% WC. This study is a preamble to the seed preservation of citrus germplasm because it allows the adaptation of drying protocols to each species and indicates the improvements needed for partially sensitive species.
Keywords: Water content; Relative humidity; Germination; Seed maturity; Citrus diversity; Seed ageing; Orthodox; Recalcitrant
Introduction
According to the recent genomic studies, allopatric evolution, hybridization and migration can explain the citrus evolution and diversification [1-3]. The main citrus crops, sweet orange (C. sinensis (L.) Osb.), lemon (C. limon (L.) Burm.), sour orange (C. aurantium L.), lime (C. aurantifolia (Christm.) Swing.), yuzu (C. junos Sieb. ex-Tan.), belonged from natural interspecific hybridizations between five ancestral taxa, citron (C. medica L.), pummelo (C. maxima Burm.) Merr.), mandarin (C. reticulata Blanco), and Papeda species such as C. micrantha Wester and C. ichangensis Swing. Trifoliate oranges (Poncirus trifoliata (L.) Raf.) and kumquats (Fortunella sp.) were also originated from Asia but due to the biology of reproduction (shift of blooming) limited the intergeneric natural hybridizations. However, the trifoliate orange is a genitor of breeding programs for production of intergeneric hybrids used as rootstocks because it remains a source of resistances to diseases and environmental stresses. The citrus rootstocks are propagated by seedling. Maintaining the viability of citrus seed over time is essential to meet the short-term (few months) conservation needs of commercial seed producers and breeders. Storage of the seeds at a relatively low temperature (5 °C) with appropriate fungicide treatment and relatively moderate drying proved adequate for short-term maintenance [4-6]. This short-term maintenance is adapted for the production of citrus rootstock seed, to bridge the gap between harvest time and spring sowing time. During the formation of seeds after fertilization, many citrus species or genotypes have the particularity of producing somatic embryos, which allow clonal propagation. Citrus rootstocks are clonally amplified by seedling of polyembryonic seeds [7]. Maintaining long-term seed viability is a necessity for ex situ conservation of citrus genetic resources, for which an effective and low-cost method of long-term conservation is needed. Many research and storage advances have been achieved predominantly in cultivated citrus species. The most promising potential long term ex situ technique appears to be cryopreservation of seeds and it is considered as the safest option to prevent seed viability loss [8,9].
Understanding behavior of seed storage depends on a knowledge of a species taxonomy, plant ecology, and seed characteristics [10]. Citrus are classified as intermediate seeded species because can withstand partial dehydration, but they cannot be stored under conventional genebank conditions because they are cold sensitive and desiccation does not increase their longevity [11,12]. Tolerance to seed desiccation varies within and between citrus species from orthodox to recalcitrant [10]. The moisture control is a key element for freezing of citrus seed [9]. The limit of citrus seeds dehydration for liquid nitrogen freezing treatment corresponds to the unfrozen Water Content (WC) in the tissue, confirming that seed survival strictly depends on avoidance of intracellular ice formation. Moisture contents between 10 to 20% (fresh weight basis) are often optimal for survival of freezing [13]. A fixed seed moisture content can be achieved by incubation of seeds in different atmospheric relative humidity controlled by salt solutions [9]. The WC equilibrium was achieved after few days or weeks of incubation, depending on species and Relative Humidity (RH). Other drying method are frequently used as laminar airflow and silica. The removal of the seed coat could permit lemon seeds to be dried to a low moisture content without any damage [14]. However, the removal of the seed coat is not necessary, if provided sufficient time is allowed for intact seeds to germinate [15]. Damage caused by low temperatures and dehydration in plant cells may be irreversible and cause cell death depending on the intensity of the stress [16]. Water is an essential element in maintaining the integrity of the cell membrane. Indeed, a water deficiency leads to a modification of the phospholipid structure of the lipid bilayer, which leads to leakage of electrolytes to the intercellular medium [17]. Many studies on seed desiccation tolerance have been down on citrus cultivars but there is considerable variability among seed lowest safe moisture content (WC) when comparing different studies [18]. In sour orange (C. aurantium), for example, viability is 25% for a WC of 13.8% [19], 40% for a WC of 14.4% [20] and 80% for a WC of 10% [8]. Similarly high discrepancies are observed in lemon (C. limon): 1% for a WC of 10% [21], 85% for a WC of 7.5% [22] and 90% for a WC of 10.3% [23]. These differences in viability between studies cannot be fully explained by the varietal diversity of these 2 species, as it has been shown that their diversification has mainly been through mutations and that human selection has mainly retained new phenotypes on fruit traits such as color, shape, size juice composition and seed-lessness [24,25]. In ancestral species, on the other hand, sexual crossing is the source of varietal diversification, generating more genetic diversity than mutation [26]. It is therefore normal to observe diversity in dried seed viability between varieties of pummelo (C. maxima) [27,28]. Various categorization of the lemon seeds was proposed by different authors from orthodox [20] and intermediate to recalcitrant [21,22]. The definition of desiccation tolerance is based on the ability of seeds not to lose viability below 10% moisture content [29]. The lower limit of the threshold water content before injuries for intermediate seeds is around 15% [30]. Most studies of desiccation tolerance were done on very few varieties at the same time and with the same conditions of seed drying, making it impossible to apply a suitable method for short or long-time conservation adapted to each citrus species. Since the control of WC is crucial for the preservation of germination capacity and viability of citrus seeds, the main objective of this study was to evaluate, the seed desiccation tolerance in a wide range of desiccation levels for the citrus species. This multi-species study has other objectives:-. The evaluation of some factors that can modify seed quality (maturity and temperature during drying), and the inheritance of desiccation tolerance across interspecific and intergeneric hybrids. The list of investigated varieties was defined to represent the citrus genetic diversity including ancestral species and cultivated groups.
Materials and Methods
Plant material
Fruits were picked on the trees of the INRAE-Cirad citrus biological resource center (San Giuliano, Corsica, France; 42° 18’ 55’ N’ and 9° 29’ 29’ E’) [31]. The seeds were extracted from maturate fruit at the ripening time of each variety (Full fruit size), washed with water and few drops of detergent, partially dried at room temperature (22-25 °C) on the open-air desk for 20h, before to be used or stored at 4 °C. Thirty-four citrus cultivars were selected to represent the four Citrus ancestral species and their interspecific hybrids such as sweet oranges, sour oranges, lemons and limes. In addition, Fortunella and Poncirus genus were represented each one by three and two cultivars respectively, completed by 2 Citrus x Poncirus hybrids usually used as rootstocks in the citrus industry, citranges (C. sinensis x P. trifoliata).
Seed desiccation
Seeds were incubated in a hermetically sealed jar containing a saturated solution of different salts in order to control the relative humidity of the atmosphere. The seeds were kept above this solution, without contact with it, on a support allowing the exchange of water molecules between the atmosphere of the jar and the seeds. The relative humidity (RH) of the atmosphere of the jar were controlled by different saturated salt solutions, NaOH (6%), KOH (9%), MgCl2 (34%), K2 CO3 (45%), NaCl (75%), NH4 Cl (78%), (NH4)2SO4 (81%), KCl (85%) and Na2 CO3 (87%) [32,33]. Seeds loose water molecules by exchange with the atmosphere until they reach equilibrium [33]. The time required for seed moisture content to stabilize depends on RH and variety [34]. To ensure MC stability, seeds were incubated for 12 days at RH levels of 6 to 45%, 20 days at RH levels of 75 and 78%, and 30 days at RH levels of 81 and 85%.
Measurement of the seed moisture (Water Content: WC)
Ten normal developed seeds were weighed (P1) and then completely dried by incubation for 16 h at 104 °C and then weighed again (P2). The water content of the seed is WC = ((P1-P2)/P1) × 100.
Germination test
Seeds were sowed on fine water saturated vermiculite in petri dishes (15x15cm), placed at 25 °C, in the dark. After 4 weeks, the number of germinated seeds with at minimum a 20mm-long emerging root axis, was scored. The germination rate (%) was then calculated.
Seed maturity
The seed weight, the fruit weight, the germination capacity and the seed moisture content were measured during 7 months from 10th of June (40-60 days after anthesis) to 10th of January (when the fruit was totally developed), for 3 genotypes usually used as rootstock, ‘Volkamer’ lemon, ‘Maroc’ sour orange, and ‘Carrizo’ citrange. The flowering time diverges between the 3 genotypes it extended from mid-April to mid- May for the ‘Volkamer’ lemon and the sour orange, but it was 2-3 weeks earlier for ‘Carrizo’ citrange. For each parameter, during the period of fruit development nine dates were chosen for sampling 8 fruits for the weight measurement, 10 seeds for weight and WC assessment, and 3x30 seeds for germination.
Effect of temperature and moisture content on seed germination during drying process and storage
Six rootstocks were selected for this experiment, three Citrus species (‘Volkamer’ lemon, ‘Rangpur’ lime and sour orange), ‘Pomeroy’ trifoliate orange (P. trifoliata) and two intergeneric hybrids, ‘Carrizo’ and ‘C35’ citranges (C. sinensis x P. trifoliata). The jars containing seeds and salt solutions for RH of 45% (K2 CO3), 75% (NaCl) and 85% (KCl), were disposed into an incubator (25 °C) or into a cold chamber (4 °C). The experiment was conducted over 270 days with samples of seeds were kept at 30, 60, 90, 120, 150, 180 and 270 days of incubation time to evaluate the WC and GR parameters.
Evaluation of the seed tolerance of citrus species against a large range of desiccation levels
The proportion of germinated seeds of 39 genotypes from different varieties, cultivars and species, was measured after drying the seeds under different RHs (85%, 81%, 75%, 62%, 45%, 23%, 6%) by using the salt solutions of KCl, (NH4)2SO4, NaCl, NH4 NO3, K2 CO3, K acetate, NaOH, respectively, at 25 °C and until the WC has reached the equilibrium. The tolerance to desiccation was evaluated by comparing the germination rate of dried seeds with the germination of untreated seeds directly sowed after extraction. Fifty seeds were used for the evaluation of germination rate for each citrus accession.
Statistical analysis
Data are expressed as mean values±SD. Differences between varieties were assessed by Duncan test and one-way analysis of variance to determine significant differences for different RH applied for seed desiccation. Statistical significance was determined with p<0.05.
Results
Evolution of germination capacity according to the seed and fruit development
Figure 1:Evolution of fruit weigh (W), seed weight, moisture (WC) and of the Germination Rate (GR) during fruit development (from 10th of June/0 to 20th of January/220 days) for ‘Volkamer’ lemon, Sour orange and ‘Carrizo’ citrange. Vertical bars represent ± SD.
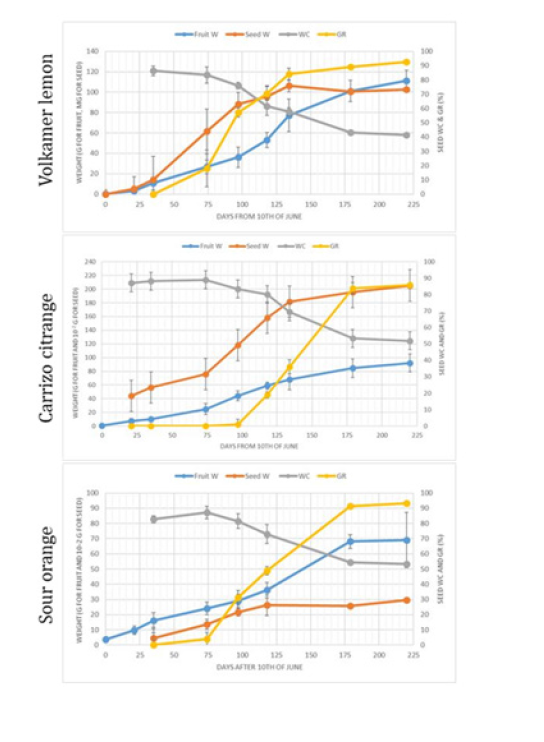
The kinetics of the seed weight, the fruit weight as well as the germination rate was similar between ‘Volkamer’ lemon, ‘Carrizo’ citrange and Sour orange (Figure 1). The kinetics of trifoliate orange seed parameters have not been plotted because they are lagged by almost 2 months due to its early flowering. In addition, no data were available from October onwards. The seed moisture content (WC) was still high (around 90%) at the beginning of the experiment (in June) and then decreased slowly from mid-September (100 days after the first measurement) until stabilization approximatively to 50% for ‘Carrizo’ citrange and sour orange and to 40% for ‘Volkamer’ lemon. During the phase of seed water loss, the seed weight always increased. Germination Rate (GR) correlated negatively with seed WC. Regardless of the variety, GR is maximum when seed WC was below 55% (it is the same with trifoliate orange). The higher germination rates were observed when the fruit and the seed weights reached their maximum. The seeds of the ‘Volkamer’ lemon had an earlier maturity of approximately 30 days compared to the two other citrus fruits (end of October versus end of November). Trifoliate orange seeds reach maturity in September. Taking into account these results on the seed maturity, the fruits of all the studied citrus fruits were harvested between December and February when they had reached their maximum size (or weight). The fruits of trifoliate orange were kept in October and seeds of kumquats in March to ensure optimal seed maturity because their bloomed in July.
The seed WC according to the species and the degree of dehydration
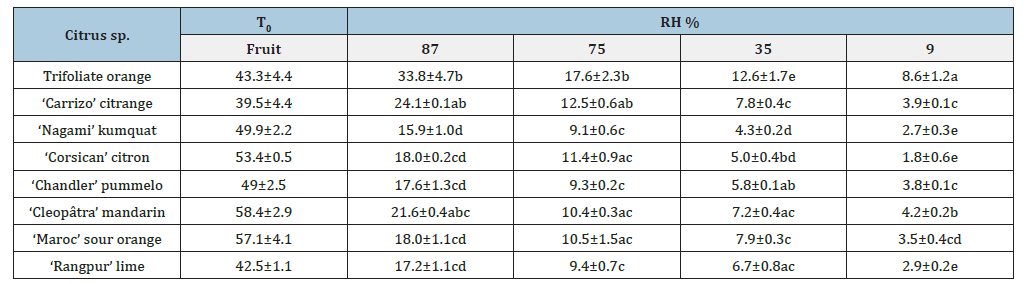
At the end of desiccation process, the equilibrated seed WC (when it reached a stable value) differed between citrus varieties (Table 1). It was always significantly higher in Poncirus trifoliata than in other rootstocks, whatever the RH. However, this difference in WC compared to other varieties decreases as RH decreases. The WC value for trifoliate orange seeds, observed at 87% RH, has not yet reached equilibrium after 47 days of desiccation, as the standard deviation is still very high (4.7). The WC values for the other varieties are stable with a standard deviation close to 1.0, they have therefore reached equilibrium. In ‘Carrizo’ citrange the equilibrated WC are significantly higher than those of Citrus species and trifoliate orange only at RH of 75% and 87%. This intermediate behavior is in agreement with its intergeneric hybrid origin (CitrusxPoncirus). The behaviors of the five Citrus species and ‘Nagami’ kumquat are similar.
Table 1:The average of final seed WC (g H2 Og-1dw) (%) and standard deviation of 8 citrus species desiccated with four relative humidity (RH in %) and just after extraction (T0 fruit). Values followed by the same letter did not differ significantly at P<0.05 using Duncan’s multiple range test.
The germination varies with the temperature, the desiccation intensity and the genotype
Figure 2:Evolution of seed germination of 6 citrus rootstocks during the desiccation period with different relative humidity (45, 75 85% RH) and two temperatures of incubation (25 °C and 4 °C). Vertical bars represent ± SD.
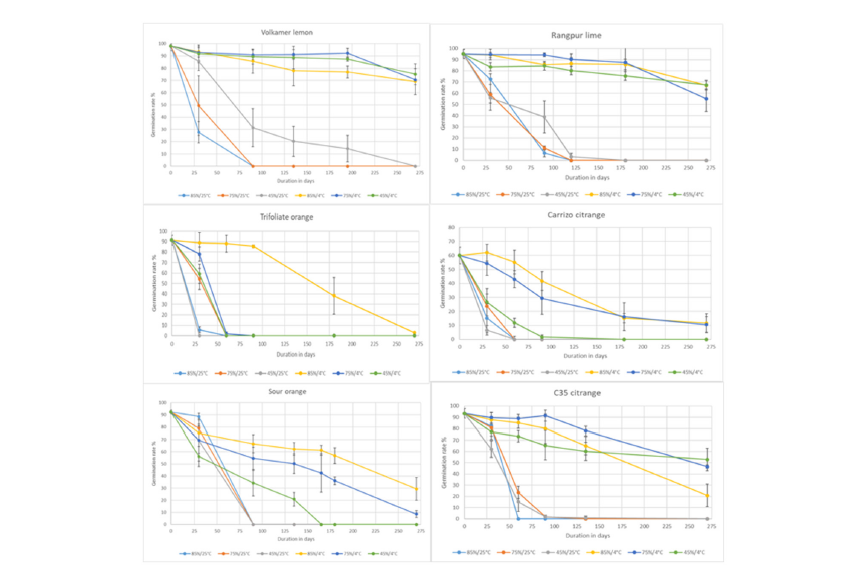
In general, and for all varieties, the highest germination rates were observed when seeds were dried at 4 °C (Figure 2). The temperature has a strong effect on the preservation of the seed germination capacity. After 100 days of incubation at 25 °C, the seeds of all the varieties do not germinate any more with the exception of the seeds of ‘Volkamer’ lemon and ‘Rangpur’ lime incubated at 45% RH their germinations are close to 30%. The germination capacity is longer preserved when the seeds were maintained at 4 °C. After 100 days of incubation in 85% RH at 4 °C, the germination was higher than 80% for ‘Volkamer’ lemon, ‘Rangpur’ lime, ‘C35’ citrange and trifoliate orange, and higher than 60% for sour orange. The germination kinetics of ‘Rangpur’ lime and ‘Volkamer’ lemon seeds during drying are very similar whatever the conditions. For ‘C35’ citrange, unlike the temperature the relative humidity does not seem to have an effect on the germination capacity. Its germination decreased and at twice as fast at 25 °C as for the previous two rootstocks and at 4 °C it was reduced to around 20-50%. For the three other rootstocks, the relative humidity has an incidence on the germination rate. At 45% RH and 4 °C, the germination capacity was null after 60 days for the trifoliate orange, 90 days for the ‘Carrizo’ citrange and 150 days for the sour orange. The seeds of these three rootstocks are not resistant to excessive drying. The seeds of trifoliate orange were even very sensitive to a RH of 75% since the germination rate is null after 60 days of incubation. After 100 days of incubation, the germination decreased by 30% for sour orange and 50% for ‘Carrizo’ citrange, with seeds incubated at 75% or 85% of RH. At the end of 9 months, the seeds of these two cultivars have a germination rate ranged between 10 and 20%. There is a difference in final water content (at equilibrium) and in the rate of decay depending on the drying temperature. At 4 °C, water loss from the seed is slowed down and the equilibrium WC value is higher than that of seeds incubated at 25 °C, regardless of relative humidity. WC values observed after 30 days at 25 °C are only reached after 90 days at 4 °C for all rootstocks. For example, in ‘Volkamer’ lemon, at 85% RH, the WC stabilizes at 11.6% after 60 days at 25 °C, whereas at 4 °C stability is achieved at 13.5% after 135 days of incubation. At 75% RH and 25 °C, the WC is already stabilized at 8.9%, while at 4 °C, the WC stability is around 10.1% and only after 90 days of incubation. Similar differences are also observed for incubation at 45% RH.
Seed tolerance against water loss during drying process performed at 4 °C
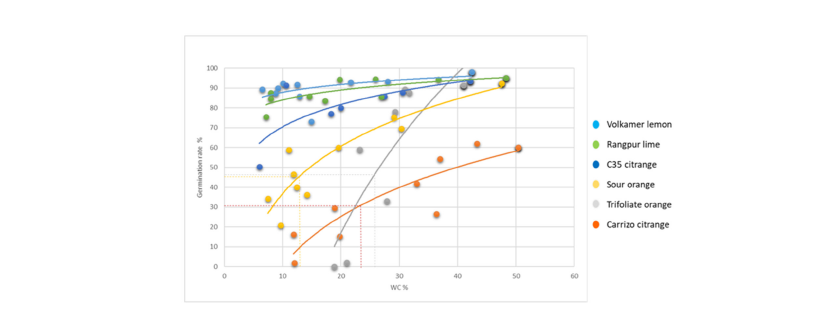
The germination rate according to the water content makes it possible to evaluate the sensitivity or the tolerance of seeds to desiccation and to measure the water content corresponding to a reduction of 50% of the germination rate (WC50). A previous experiment had shown that the time required to achieve WC stability during desiccation was long at high relative humidity (>20 days) and varied depending on the species [34]. Therefore, only the data obtained from incubation made at 4 °C and during the first 160 days were used to avoid or limit the effect of the seed ageing (Figure 3). The evolution of germination rater as a function of WC of ‘Volkamer’ lemon and ‘Rangpur’ lime was almost linear with quite no slope. The decrease in germination rate between the highest (42%) and lowest (7%) WC was only about 10% of the initial value for these two Citrus species. The germination rate of the ‘C35’ citrange decreased steadily but gently during the decrease of WC and the total reduction in water content was only 25% at the lower seed WC. The curves of sour orange and ‘Carrizo’ citrange evolved similarly with a drastic slowdown of the germination rate. The curve corresponding to the trifoliate orange presented a very inclined slope with a very pronounced decrease of the germination rate when the WC was below 30%. The graphical estimation of the WC50 from these curves could only be evaluated for these last three citrus genotypes because only they showed a significant decrease in germination rate. The WC50 was graphically estimated around 13% for sour orange, 24% for ‘Carrizo’ citrange and 27% for trifoliate orange. It should be noted that the initial germination rate of ‘Carrizo’ citrange seeds was low (60%) in contrast to other citrus rootstocks which were at about 90%. It is thus preferable to evaluate the level of sensitivity of seeds to desiccation in relative proportions of decrease of the germination rate rather than on absolute values. In agreement with the WC50 values and the curves of germination rate versus water content, it was concluded that trifoliate orange and ‘Carrizo’ citrange are the most sensitive varieties to desiccation, sour orange less sensitive, ‘C35’ citrange tolerant, ‘Rangpur’ lime and ‘Volkamer’ lemon highly tolerant.
Figure 3:Kinetics of the Germination Rate (GR) and the seed WC (% of fresh matter weight) for six citrus rootstocks, made only with values obtained at 4 °C and the period of the first 160 days of incubation. The dotted lines in tendency curves indicate the water content (WC50) corresponding to half reduction of the initial germination rate (50% loss of GR) for the citrus genotypes presenting a sufficient GR decrease.
Evaluation of the diversity of seed desiccation tolerance of citrus species
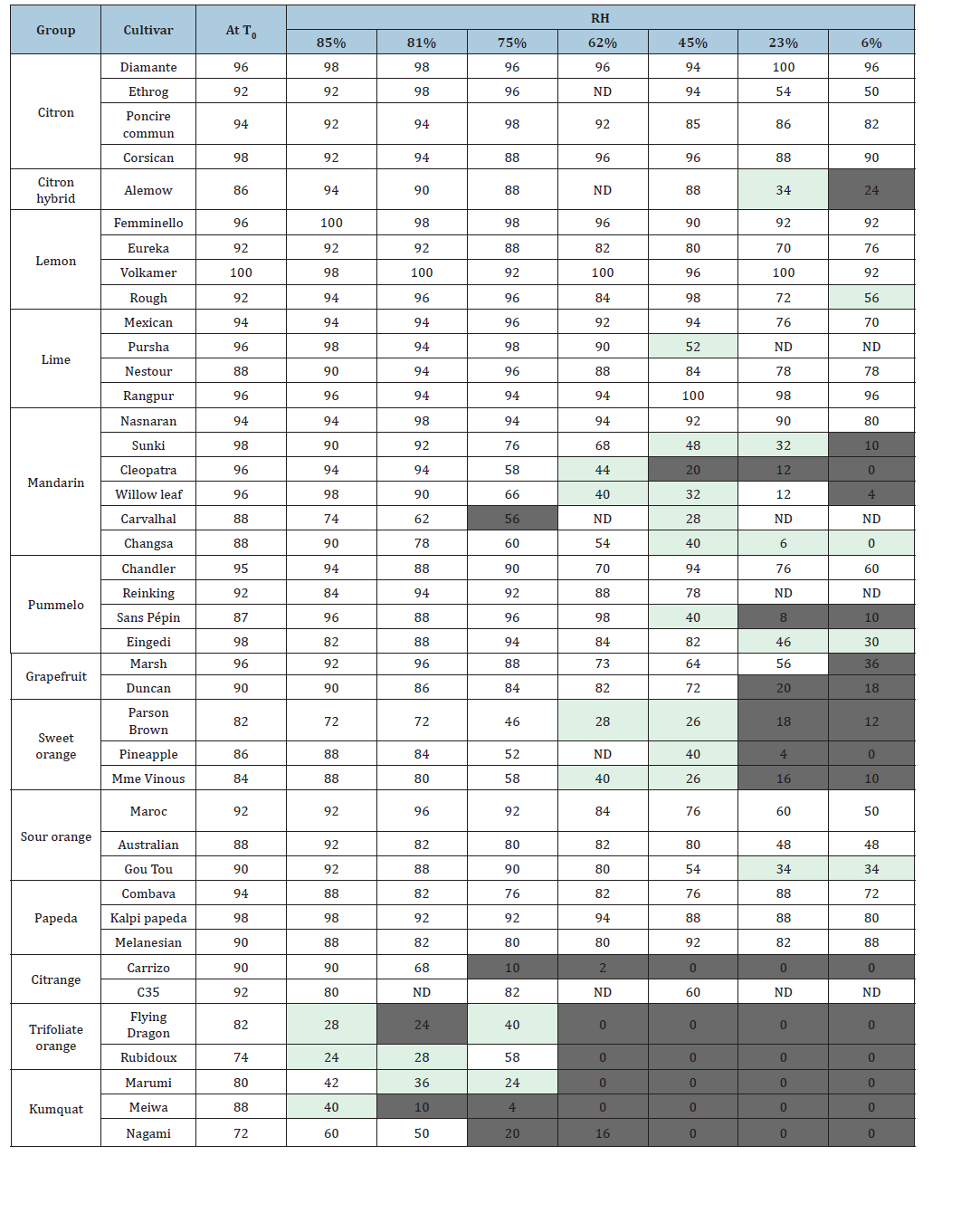
Each species was represented by 2-4 cultivars to assess the seed
behavior against a range of desiccation degrees for each taxonomic
group (Table 2). The initial germination rate when the seeds
were sown just after extraction, was high (>72%) for all studied
cultivars. In general, there was little or no variation between
cultivars of the same species. Given the absence of repetition (only
one measurement carried out on 50 seeds), the variation between
GR lower than 15% are not taken into account and only relative
decreases higher than 30% are considered as informative for the
qualification of the tolerance status to desiccation conditions of each
citrus cultivar. The four ancestral species of the genus Citrus showed
variable behavior with notably tolerance to low RH (6% and 23%
corresponding to a range of 3.5-7.5% WC) for all citrons (C. medica)
and Papedas that maintained a viability higher than 80% (excepted
for Combava). Pummelos (C. maxima) were also tolerant (more
than 50% of GR decreases were observed only from 23% and lower
RH). Among ancestral species, mandarins (C. reticulata) were the
most sensitive with a loss close to 50% of germination at 62% RH
(the WC is close to 10% for 62% RH). Kumquats (Fortunella sp.) lost
more than 50% of germination at 75% RH while trifoliate oranges
(P. trifoliata) lost the same proportion at 81% RH. Limes and lemons
displayed generally the same tolerance level as their citron parent
except for ‘Alemow’ (C. macrophylla) which lost more than 50% of
germination between 23% and 6% RH. The germination capacity
of sour oranges was maintained high at 45% RH while grapefruits
have a behavior comparable to their pummelos parent, as well as
sweet oranges had a behavior close to that of mandarins. Intervarietal
diversity can be observed in few species based on seed
behavior at low RH (or high drying conditions). This is the case for
pummelos, mandarins, sour oranges and citranges. ‘Cleopatra’ and
‘Changsa’ mandarins are more sensitive than ‘Sunki’ with a decrease
of the germination at 45% RH, representing 79, 59 and 26% of the
initial rate, respectively. The germination of ‘Sans Pepin’ pummelo
decreases drastically at 45% RH (53% of loss), while it is still very
high for the other two cultivars (>80%). The behavior of ‘C35’ and
‘Carrizo’ citranges was opposite with a high sensitivity of ‘Carrizo’.
Despite this intra specific variability, a classification of species
from the most sensitive to the most tolerant to seed desiccation
can be proposed: trifoliate oranges_<_kumquats<‘Carrizo’
citrange_<_sweet oranges<mandarins<‘C35’citrange<grapefruits<
pummelos_<_sour oranges_<‘_Alemow’_<_lemons_<papedas–
limes–‘Nasnaran’_<_citrons.
Table 2:Percentage of germination (GR) of citrus cultivars and species with seeds incubated in a range of RH. T0 represents the germination rate of untreated seeds directly sown after extraction from fruit, the grey color and its intensity represents the diminution of GR relatively to the T0: light grey for decreases between 30% - 50%, medium grey 50%-70% and deep grey for higher than 70%.
Discussion
Seed quality and maturity
Three main factors important to seed storage are moisture content, temperature and seed quality. Among these factors, seed quality is an ambivalent and complex factor because it is subject to environmental and genetic controls [35]. Most often, seed lots are of heterogeneous quality, often due to differences in flowering period, dry matter accumulation, dormancy, and maturation date, all of which are genetically regulated characteristics that affect seed longevity [36]. Seed quality, in terms of sensitivity to desiccation, has been suggested as the reason for the variability observed in wild Coffea arabica species [37]. Seed development initiates by embryogenesis followed by an embryogenic morphogenesis. After that, developing seeds enter in a maturation stage, corresponding to reserve accumulation, by reorganization of metabolism and synthesis of storage compounds (starch, storage protein and oil). Finally, the desiccation stage is the last step of seed maturation [38]. Seed desiccation plays a role in redirecting metabolism from developmental to a germinative mode by inducing hydrolytic enzymes essential to the growth of seedling [39]. Seed desiccation is an active stage in terms of gene expression and metabolism [40]. The transition of developing seeds from the phase of reserve accumulation to desiccation is associated with distinct gene expression and metabolic switches. Maximum desiccation tolerance is often acquired at mass maturity (max. dry weight accumulation) [12,39]. The use of seeds that have not reached their maximum maturity and therefore tolerance to desiccation could explain the contradictory results in the literature on seed responses to desiccation [41]. Our results are in agreement with this description of seed maturation by the increase of germination capacity related to the water loss. This desiccation phase is therefore necessary for the seed to acquire maximum germination capacity. The time required for the seeds to reach full maturity (>80%) depends on the variety, with nearly 45 days separating the earliest (‘Volkamer’ lemon) from the latest (sour orange) even though flowering occurs at the same time for both species. Once the fruits have reached maturity, the water content of the seeds and the germination rate no longer vary during the fruit harvesting period [42]. These stages of seed development are often identified using non-destructive visual maturity indicators such as the size, color, and hardness of the fruit or seeds [43,44]. To obtain seeds with uniform maturity, which is essential for comparing and interpreting desiccation tolerance capacities, it is useful to identify maturity markers. Because the fruits of citrus trees used as rootstocks are not consumed, it is difficult to determine indicators of fruit ripeness. In general, there is a good correlation with abscission [44]. The easiest indicator to observe is the size (or mass) of the fruit. When it exceeds 85% of its final weight, the seed germination reaches its maximum (> 90%) regardless of the variety. The water content of the seeds in the fruit is also a good indicator of seed maturity. The germination is highest when it is close to or below 55%. Water content was the indicator used in subsequent experiments to ensure optimal seed maturity before treatment.
Fluctuation of water exchanges between seeds and the atmosphere
The deadline to reach a stable WC (cessation of water exchange between seeds-atmosphere-salt solution) varies according to the citrus species, suggesting different water flow regulation through the membrane of seed teguments. Seed coats are often suggested as a contributing factor (chemical or physical inhibitors) in the erratic, slow and reduced germination in response to desiccation and storage that has been observed in citrus seeds [14,45,46]. The development of physical dormancy via a seed coat barrier to water uptake and gas exchange [47]. Crane [48] suggested such interactions may account for the damage observed in intermediate species on long-term storage [48]. Citrus seeds have characteristically high oil content, between 37% to 52% lipid content [9]. Therefore, this phenomenon could contribute to the variable and unusual seed physiology observed in citrus. Thus, analysis of seed oils may allow more accurate interpretation of survival responses in citrus species after drying.
Seed ageing
Seed aging is a natural process that causes a decrease in seed viability and vigor over time, reducing their ability to germinate. This phenomenon is of great concern to plant breeders and has implications for the preservation of plant genetic resources. Physiologically, aging seeds undergo a series of complex biochemical changes, including lipid peroxidation, protein oxidation, DNA damage, and changes in the antioxidant system [49]. This decline is caused by various factors, including the accumulation of Reactive Oxygen Species (ROS), changes in the composition and integrity of membrane lipids, and alterations in gene expression and protein synthesis [50]. The accumulation of ROS causes them to react with unsaturated fatty acids and causes changes in cell membranes, such as lipid peroxidation and, ultimately, their destruction [51]. Enzymatic detoxification and cell membrane repair are the main ways to delay ageing [49]. The activity of ROS-trapping enzymes and non-enzymatic antioxidant enzymes gives seeds high resistance to oxidative damage and minimizes damage to cells [52]. No studies have yet been conducted on the molecular changes that occur during the ageing of citrus seeds that could explain the rapid loss of germination when seeds are stored at room temperature. In our study, seed ageing is particularly accelerated in trifoliate oranges (P. trifoliata). This is evident early on, as germination rates are low for high water contents (81 and 85%) because the time taken to reach equilibrium for WC is close to 50 days at 25 °C (data not shown), whereas at 75% RH, WC equilibrium is reached after only 25 days. Germination is therefore higher at this RH. Low temperatures (4 °C), even if they slow down the flow of water between seeds and the atmosphere, help to limit the ageing phenomenon.
Diversity of seed response to desiccation in the citrus family
It is well known that seeds of most citrus species are not orthodox and that their long-term storage under conventional conditions (low seed water content and at freezing temperatures) is not possible [10]. Nevertheless, citrus species show high variability in storage responses, some are desiccation tolerant and others have complex intermediate seed storage behavior, with some loss of viability at lower moisture contents [10]. Lime (Citrus aurantifolia), lemon (C. limon), Rough lemon (C. jambhiri) and grapefruit (C. paradisi) are the most tolerant species; at low water contents (<10%), they maintain germination rates above 80% [9,20,53]. In contrast, ‘Calamondsi’ (C. madurensis), ‘Savage’ citrange, ‘Sacaton’ citrumelo, trifoliate orange (P. trifoliata) and mandarin (C. reticulata) are known to be sensitive to seed desiccation [9,27,54]. However, there are also many discrepancies between different studies. For example, for sour orange (C. aurantifolia) seeds with water contents between 10 and 14%, the germination fluctuates between 40 and 80% [8,20]. Even greater variations are observed in lemon (C. limon), where the germination varies from 1 to 85% for water contents of 10 and 7.5% respectively [21,22]. It is therefore difficult to make comparisons between different studies because drying conditions vary between studies (coated or uncoated seeds; drying using air flow, silica, atmosphere with controlled relative humidity, temperature). Germination conditions could also be a cause of variation. For example, lemon (C. limon) seeds needed a higher germination temperature (i.e. 30 °C) and a longer germination time (5 weeks) post drying [15]. Many germination tests in citrus had previously been conducted over shorter time periods and at a lower temperature (e.g. 20 ºC) [15]. This may be a factor involved (i.e. insufficient time for germination) in some of the variable reports of desiccation sensitivity in citrus. A longer time for water uptake is suggested as the reason for the resultant delay in germination on drying of citrus seeds [46]. In our study, where seed maturity is controlled and drying and germination conditions are identical for all varieties and species, we do not observe any fluctuations between clonal varieties of the same species. The varietal diversity of most secondary species cultivated for fruit consumption is based solely on the clonal selection of somatic bud mutations identified in orchards. This applies, among others, to lemon trees (C. limon, [24]), orange trees (C. sinensis, [55]), and grapefruit trees (C. paradisi, [56]). For each species, cultivars have the same level of tolerance to desiccation. Another critical point could be the classification of citrus fruits and the use of a variety as a reference for a species. For example, in sour oranges (C. aurantifolia), there is genetic variability linked to hybridization involving different parents [25]. The ‘Gou Tou’ and ‘Australian’ varieties do not have the same genetic origin and therefore do not have the same genotype as the ‘Maroc’ variety, for example. The tolerance of their seeds to desiccation may therefore vary. Lemons are another example of genetic diversity, with varieties of diverse genetic origins. The ‘Femminello’ and ‘Eureka’ varieties are cultivars for consumption. They have the same genetic origin, a hybridization between a sour orange and a citron [1]. They differ only in mutations identified by clonal selection. The ‘Volkamer’ and ‘Rough’ lemons, used as rootstocks, are the result of crosses between citrons and mandarins [24]. Although genetically different, these lemon varieties have the same high level of drought tolerance.
The inheritance of tolerance to low water content
Species, C. aurantifolia, C. macrophylla, C. limon, C. jambhiri, C. karna, and C. limonia share citron as a common ancestor [24]. Although some of them have a parent that is sensitive to low water content (mandarin and sour orange), they have inherited the drought tolerance of C. medica. This trait may be dominant in this ancestral species. The example of citranges is slightly different. ‘Carrizo’ is thought to have inherited the sensitivity of its pollinating parent, the trifoliate orange (P. trifoliata) while, ‘C35’ citrange is more tolerant to desiccation than its other parent, the sweet orange. This is therefore a case of transgressive inheritance. ‘Nasnaran’ mandarin is very tolerant to desiccation. But in reality, this is a hybrid between mandarin and a Papeda (C.micrantha, not present in this study) [57]. His tolerance is therefore a legacy from his Papeda progenitor. Other secondary species related to C. reticulata differ in their level of tolerance. Sour oranges (C. aurantium) have a tolerance equivalent to that of pummelo, while oranges (C. sinensis) inherited the sensitivity of mandarins. The pummelo (C. maxima) has transmitted its tolerance to the grapefruit (C. paradisi), which is a cross between C. sinensis and C. maxima [1].
Conclusion
To impartially assess the tolerance of seeds to different degrees of desiccation, it is first necessary to ensure the quality of the seeds, i.e., their optimum germination capacity. The time required to reach this maturity varies depending on the species and can be assessed by the water content of the seeds, which must be less than 55%. To prevent accelerated aging, seeds must be dried at 4 °C or 25 °C, but in the latter case for a short period (21 days). Seed tolerance to desiccation varies considerably depending on the species. All varieties of citrons and their hybrids (lemons and limes) have a high tolerance to low water content (~5%) and their seeds can be considered as orthodox. Germination capacity decreases with seed water content in grapefruit, pummelos, and sour oranges, so their tolerance is moderate, while seeds of sweet oranges and mandarins are recalcitrant (sensitive to water content close to 15%). The most sensitive species are kumquats (Fortunella sp.) and trifoliate oranges (P. trifoliata), which lose more than 50% of their germination capacity at water contents close to 25%. Tolerant species can be included in the seed cryopreservation program using the desiccation methods developed in this study.
Acknowledgment
Thanks to citrus Biological Resource Center (BRC) though the person of Dr Olivier Pailly for the availability of biological material. We thank Dr Stéphane Dussert from IRD Montpellier France for helpful discussions. This work was initiated with the CRYOVEG project supported by grants from IBISA. This work has received research funding from the French government managed by the National Research Agency under the France 2030 program, with reference number ANR-22-EXES-0016.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu GA, Prochnik S, Jenkins J, Salse J, Hellsten U, et al. (2014) Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus Nat Biotechnol 32(7): 656-662.
- Wu GA, Terol J, Ibanez V, Lopez GA, Perez RE, et al. (2018) Genomics of the origin and evolution of Citrus. Nat Cell Biol 554(7692): 311-316.
- Ollitrault P, Curk F, Krueger R (2020) Citrus In: Talon WP, Caruso M, Gmitter M (Eds.), The genus Citrus. UK, pp. 57-81.
- Mungomery WV, Agnew GWJ, Prodonoff ET (1967) Maintenance of citrus seed viability. J Agrc Animal Sci 23: 103-120.
- Soost RK, Cameron JW (1975) Citrus. In: Janick J, Moore JN (Eds.), Advances in fruit breeding. Purdue University, USA, pp. 507-540.
- Mobayen RG (1980) Germination and emergence of citrus and tomato seeds in relation to temperature. Journal of Horticultural Science 55: 291-297.
- Castle WS (1987) Citrus In: Rom RC, Carlson RF (Eds.), Rootstocks for fruit crops. USA, pp. 61-399.
- Lambardi M, De Carlo A, Biricolti S, Puglia AM, Lombardo G, et al. (2004) Zygotic and nucellar embryo survival following dehydration/cryopreservation of Citrus intact seeds. CryoLetters 25(2): 81-90.
- Hor YL, Kim YJ, Ugap A, Chabrillange N, Sinniah UR, et al. (2005) Optimal hydration status for cryopreservation of intermediate oily seeds: Citrus as a case study. Ann Bot 95(7): 1153-1161.
- Hong TD, Ellis RH (1995) Interspecific variation in seed storage behaviour within two genera-Coffea and Citrus. Seed Science and Technology 23: 165-181.
- Ellis RH, Hong TD, Roberts EH (1990) An intermediate category of seed storage behaviour? I Coffee. Journal of Experimental Botany 41(9): 1167-1174.
- Vertucci CW, Farrant JM (1995) Acquisition and loss of desiccation tolerance. In: Kigel J, Galili G (Eds.), Seed development and germination. USA, pp. 237-271.
- Engelmann F (2004) Plant cryopreservation: Progress and prospects. In Vitro Cell & Dev Biol-Plant 40: 427-433.
- Mumford PM, Grout BWW (1979) Desiccation and low temperature (-196 °C) tolerance of Citrus limon seeds. Seed Science and Technology 7: 407-410.
- King MW, Roberts EH (1980) The desiccation response of Citrus limon L seeds. Annals of Botany 45: 489- 492.
- Kacperska A (2004) Sensor types in signal transduction pathways in plant cells responding to abiotic stressors: Do they depend on stress intensity? Physiologia Plantarum 122: 159-168.
- Leopold AC, Vertucci CW (1989) Moisture as a regulator of physiological reaction in seeds. In: Phillip CS, Miller BMD (Eds.), Seed Moisture. USA, pp. 51-67.
- Hamilton KN (2007) Exsitu conservation of Australian citrus species: Investigation of seed biology, cryopreservation and in vitro culture.
- Khan MM, Akhtar AM, Abbas M, Iqbal MJ (2003) Studies on seed desiccation tolerance in four citrus Pak J Agri Sci 40(1-2): 55-62.
- King MW, Soetisna U, Roberts EH (1981) The dry storage of citrus An Bot 48: 865-872.
- Marques A, Nijveen H, Somi C, Ligterink W, Hilhorst H (2019) Induction of desiccation tolerance in desiccation sensitive Citrus limon seeds. Journal of Integrative Plant Biology 61 (5): 624-638.
- Hong T, Ahmad N, Murdoch A (2001) Optimum air-dry storage conditions for sweet orange (Citrus sinensis (L) Osbeck) and lemon (Citrus limon (L) Burm f) seeds. Seed Sci Technol 29: 183-192.
- Orjuela PJM, Graivera N, Victoria SMV, Zaritzky NE (2019) Effect of the desiccation tolerance and cryopreservation methods on the viability of Citrus limon L Burm cv Eureka seeds. Cryobiology 89: 51-59.
- Curk F, Ollitrault F, Garcia LA, Luro F, Navarro L, et al. (2016) Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Annals of Botany 117(4): 565-583.
- Ferrer V, Costantino G, Paoli M, Paymal N, Quinton C, et al. (2021) Intercultivar diversity of sour orange (Citrus aurantium l) based on genetic markers, phenotypic characteristics, aromatic compounds and sensorial analysis. Agronomy 11(6): 1084.
- Ollitrault P, Luro F (2001) Citrus. In: Charrier A, Jacquot M, Hamon S, Nicolas D (Eds.), Tropical plant breeding. France, pp. 55-77.
- Saipari E, Goswami AM, Dadlani M (1998) Effect of seed drying on germination behaviour in Citrus. Scientia Horticulturae 73(2-3): 185-190.
- Wen B, Cai C, Wang R, Tan Y, Lan Q (2010) Critical moisture content windows differ for the cryopreservation of pomelo (Citrus grandis) seeds and embryonic axes. CryoLetters 31(1): 29-39.
- Alpert P (2005) The limits and frontiers of desiccation‐tolerant life. Integr Comp Biol 45(5): 685-695.
- Walters C (2015) Orthodoxy, recalcitrance and in-between: Describing variation in seed storage characteristics using threshold responses to water loss. Planta 242(2): 397-406.
- Luro F, Bloquel E, Tomu B, Costantino G, Tur I, et al. (2017) The INRA-CIRAD citrus germplasm collection of San Giuliano, Corsica. pp. 243-261.
- Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards-A Physics and Chemistry 81: 89-96.
- Dussert S, Engelmann F, Louarn J, Noirot M (2004) Inheritance of seed desiccation sensitivity in a coffee inter-specific cross: Evidence for polygenic determinism. Journal of Experimental Botany 55: 1541- 1547.
- Luro F, Tur I, Pailly O (2024) Development of a seed cryobank to preserve the citrus genetic resources of INRAE-Cirad germplasm: A complementary strategy to the tree field conservation. pp. 6-11.
- Walters C (2003) Optimizing seed banking procedures. In: Smith RD, Dickie JD, Linington SH, Pritchard HW, Probert RJ (Eds.), Seed conservation: Turning science into practice. UK, pp. 724-743
- Walters C (1998) Understanding the mechanisms and kinetics of seed aging. Seed Science Research 8: 223-244.
- Vasquez N, Salazar K, Anthony F, Chabrillange N, Engelmann F, et al. (2005) Variability in response of seeds to liquid nitrogen in wild coffee (Coffea arabica L). Seed Science and Technology 33: 293-301.
- Kermode AR, Derek BJ (1985) The role of maturation drying in the transition from seed development to germination: III Insoluble protein synthetic pattern changes within the endosperm of Ricinus communis L seeds. J Exp Bot 36(2): 1928-1936.
- Kermode AR, Finch SBE (2002) Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In: Black M, Pritchard HW (Eds.), Desiccation and survival in plants: Drying without dying. CABI Digital Library, pp. 149-184.
- Angelovici R, Galili G, Fernie AR, Fait A (2010) Seed desiccation: A bridge between maturation and germination. Trends in Plant Science 15(4): 211-218.
- Daws MI, Cleland H, Chmielarz P, Leprince O, Mullins CE, et al. (2006) Variable desiccation tolerance in acer pseudoplatanus seeds in relation to developmental conditions: A case of phenotypic recalcitrance? Functional Plant Biology 33(1): 59-66.
- Orbovic V, Dutt M, Grosser JW (2013) Evaluation of the germination potential of Citrus seeds during the harvesting season. Hortscience 48(9): 1197-1199.
- Hay FR, Smith RD (2003) Seed maturity: When to collect seeds from wild plants. In: Smith RD, Dickie JD, Linington SH, Pritchard HW, Probert RJ (Eds.), Seed conservation: Turning science into practice. UK, p. 1023.
- Carvalho DU, Boakye DA, Gast T, Leite RP, Alferez F (2021) Determining seed viability during fruit maturation to improve seed production and availability of new Citrus Front Plant Sci 12: 777078.
- Mumford PM, Panggabean G (1982) A comparison of the effects of dry storage on seeds of Citrus Seed Science and Technology 10: 257-266.
- Soetisna U, King MW, Roberts EH (1985) Germination test recommendations for estimating the viability of moist and dry seeds of lemon (Citrus limon L Burm) and lime (Citrus aurantifolia (Christm) swing. Seed Science and Technology 13: 87-110.
- Matilla A, Gallardo M, Puga HMI (2005) Structural, physiological and molecular aspects heterogeneity in seeds: Review. Seed Science Research 15(2): 63-67.
- Crane J, Kovach D, Gardner C, Walters C (2006) Triacylglycerol phase and ‘intermediate’ seed storage physiology: A study of Cuphea carthagenensis. Planta 223: 1081-1089.
- Kavadala JB, Patel MB, Parmar PK, Patil K (2023) Seed ageing physiological, biochemical and molecular basis: A review. The Pharma Innovation Journal 12(4): 1511-1517.
- Zamani A, Sadat NSA, Tavakol AR, Iran NH, Ali AG, et al. (2010) Lipid peroxidation and antioxidant enzymes activity under natural and accelerated ageing in safflower (Carthamus tinctorius L) seed. Iranian J Agric Sci 41: 545-554.
- Pukacka S, Ratajczak E (2005) Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J Plant Physiol 162: 873-885.
- Tabatabaei SA (2015) The changes of germination characteristics and enzyme activity of barley seeds under accelerated ageing. Cerc Agron Mold 48: 61-67.
- Khan MM, Thompson K, Usman M, Fatima B (2002) Role of moisture content and controlled atmosphere in Citrus seed storage. International Journal of Agriculture & Biology 4(2): 259-266.
- Khan MM, Akhtar AM, Abbas M, Iqbal MJ (2003) Studies on seed desiccation tolerance in four citrus species. Pak J Agri Sci 40(1-2): 55-62.
- Ferrer V, Paymal N, Costantino G, Paoli M, Quinton C, et al. (2023) Correspondence between the compositional and aromatic diversity of leaf and fruit essential oils and pomological diversity of 43 sweet oranges (Citrus x aurantium var sinensis L.). Plants 12(5): 990.
- Luro F, Marchi E, Costantino G, Paoli M, Tomi F (2025) Diversity of pummelos (Citrus maxima (burm.) merr.) and grapefruits (Citrus x aurantium paradisi) inferred by genetic markers, essential oils composition, and phenotypical fruit traits. Plants 14(12): 1824.
- Ollitrault P, Terol J, Garcia LA, Bérard A, Chauveau A, et al. (2012) SNP mining in C clementina BAC end sequences: Transferability in the Citrus genus (Rutaceae), phylogenetic inferences and perspectives for genetic mapping. BMC Genomics 13:13.
© 2025 François L. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






