- Submissions

Full Text
Biodiversity Online J
Predation and Dispersal: A Review of how Limnoperna fortunei (Dunker, 1857) Changes Trophic Relationships in Invaded Environments
Davi VC1,2, Mariana PR1, Amanda MSM1,3, Erico TFF1,4, Daniel MR1, Antonio VC1,5 and Rayan SDP1,3*
1Center for Bioengineering of Invasive Species in Hydroelectric Plants (CBEIH), Brazil
2Department of Genetics, Federal University of Minas Gerais (UFMG), Brazil
3Department of Morphology, Federal University of Minas Gerais (UFMG), Brazil
4Materials Science and Engineering, Michigan Technological University, USA
5School of Design, State University of Minas Gerais (UEMG), Brazil
*Corresponding author:Rayan Silva de Paula, Federal University of Minas Gerais, Belo Horizonte-MG, 31270-901, Brazil
Submission: January 25, 2025; Published: May 01, 2025

ISSN 2637-7082Volume5 Issue 3
Abstract
Limnoperna fortunei, commonly known as the golden mussel, is a highly invasive freshwater bivalve that
has spread extensively across South America, causing significant ecological and economic disruptions.
Its invasion alters native biodiversity, reshapes trophic interactions, and generates costly challenges for
hydroelectric power plants and other industries. To address these impacts, understanding the ecological
and biological mechanisms underlying the golden mussel’s invasion is crucial. This review examines
three key aspects of L. fortunei’s role in invaded ecosystems
a) Its impact on food webs through the establishment of novel ecological relationships, including
competition, predation, and commensalism.
b) The development of new predator-prey dynamics involving fish, freshwater turtles, crabs, and
hydrozoans.
c) Its dispersal mechanisms, which are facilitated by human activities such as fishing, boating, and
shipping, as well as animal-mediated transport.
By synthesizing current knowledge, this review underscores the importance of targeted prevention, control strategies, and public outreach to mitigate the spread and impacts of L. fortunei, emphasizing the need for further research to address knowledge gaps in its ecological interactions and dispersal.
Keywords:Biological invasion; Ecosystem; Ecological interactions; Environmental impacts; Food web; Golden mussel; Hydroelectric power plants; Invasive species; Freshwater; Limnoperna fortunei
Introduction
Limnoperna fortunei, known as the golden mussel, is a freshwater bivalve mollusk belonging to the Mytilidae family and an invasive species which can be found in South America. It was likely introduced by ballast water through the intense boat traffic between Southeast Asia and South America in 1989 (first record at the La Plata River, Argentina), and has impacted the invaded environments in several ways, from biotic diversity to industrial environments such as hydroelectric power plants [1]. The golden mussel is gonochoric with external fertilization and has a rapid growth which increases its development at high temperatures, 25 °C to 28 °C, which makes it well suited to the South American climate. Some larval stages are planktonic, that is, they can be carried away by the current, and this is key to the natural dispersion of the golden mussel [2]. The byssus thread are filaments produced in glands localized in the golden mussel’s foot, which aid biofouling ability. This feature starts to be produced during the pediveliger stage and continues into the adult stage, once the adult stage has been reached the golden mussel changes its behavior from planktonic to benthic [3]. This change in their lifecycle allows the golden mussel to fix in a lot of solid substrates such as rocks, trunks, other mollusks shells, boat hulls, fish nets, glass plates and teflon and hence, causes complications in the pipelines of hydroelectric power plants. Even the golden mussel being benthic in the pediveliger and adult stages, there are reports that it can crawl with its foot on substrate and reattach in new locations after an environmental disturbance [1]. Another factor in the success of the golden mussel when invading a new environment, is the great protection offered due to its shell, which is a protective structure made of calcium carbonate (CaCO3). It is composed of two valves that, depending on the situation, can open or close voluntarily, helped by adductor muscles which bind the visceral mass to each one of these valves.
According to Nakamura-Filho [4], the shell confers a mechanism of resistance for the golden mussel and it is composed by a periostracum, which is the most external layer, and three other layers: calcite layer also known as the cloud layer, nacreous layer and aragonite layer, being calcite and aragonite the two forms that CaCO3 can occur in nature. Considering these factors, the shell confers a resistance for golden mussel in the environment besides some advantages, such as difficult digestion by non-specialized predators (they cannot crush the shell and digest the visceral mass), resistance against stressing environment, and the capacity to open and close to avoid the desiccation when it is outside the water [5,6]. After present these fundamental characteristics about the golden mussel, is possible to understand how it can generate multiples consequences in the ecosystem such as changes the diet of native species, larvae block the inhalant channel of other bivalves, increases non-common populations like malacophagus animals, being able to extinguish endemic species, promotes blooms of toxic cyanobacteria, and causes fouling in pipelines of hydroelectric power plants [4]. According to Adelino [7], the only aquatic invasive species that has a significant cost in Brazil is the golden mussel, which costs around US $10 million/year. The costs are concentrated in prevention, control, repair of damages resulting from the invasion, and research activities. Aside from the aforementioned implications involving hydroelectric power generation, the link between the golden mussel and the environment as well as the characteristics that favor its dispersal must be also understood. With further research in this area, it is hoped that novel strategies that deal with its impacts can be developed. In this review we discuss the versatility of the golden mussel in terms of its dispersal specifically relating this with anthropic activities.
Role in food web
The golden mussel is a filtering species with adapted gills that allow it to retain particulate matter suspended in the water column. This species feeds on a variety of suspended organisms and particles, such as bacteria, phytoplankton, microzooplankton and dissolved organic material [8]. The water enters through an opening in the posterior region, called an inhalant syphon, and into the mantle cavity where it is directed to the gills, that are flat, double lamellar and composed of several juxtaposed filaments structures [9]. The captured particles are surrounded by mucus and guided towards the marginal food groove, located on the free margin of the gills (Figure 1a). In the marginal food groove, the material is transported, in mucous cords, to the region of the labial palps [10]. The smooth region of the palps receives the particles and directs them to the region with grooves, where classification takes place (Figure 1b). The particle screening mechanism depends not only on the particle size but also on the physicochemical properties of the filtered material [10]. The alimentary canal has the ciliated oesophagus, which opens into the stomach located on the anterodorsal margin of the shell (Figure 2). The digestive gland opens in the stomach through various ducts, releasing digestive enzymes in the process. Particles rejected from the stomach as well as waste from the active digestion gland pass into the intestine, which end up in anus and faeces are also released through the exhaling opening [8]. Rejected particles are transported, as opposed to ingested, to the mantle cavity. These particles are attached to mucus and called pseudofaeces. They are expelled from ciliary currents in the mantle, together with water, until another opening present in the posterior region, called the exhalant syphon [8].
Figure 1:Palpe of the Limnoperna fortunei.
a) Ventral perspective of the gills (g) and palp (p) in Scanning Electron Microscopy.
b) Lateral perspective of one palp showing its smooth region (s) and region with grooves (gr). The arrow shows
the mucous cord attached to the palp.

Figure 2:Gastrointestinal tract of Limnoperna fortunei. Light microscope images: in
a) Longitudinal section of the alimentary canal of L. fortunei. The arrow shows the mouth region.
b) Cross-section of the Alimentary Canal (AC).
c) The lining epithelium of the alimentary canal and the asterisk shows the presence of cilia on its surface.

The rate of filtration by the golden mussel is higher than other invasive bivalves such as Dreissena polymorpha, the zebra mussel, and Corbicula fluminea [11]. Therefore, the golden mussel is able to intensively modify the zooplankton and phytoplankton of an invaded ecosystem [11]. Studies in this area are still scarce but current research obtained significant results in the correlation between the golden mussel and an increase in populations of toxic cyanobacteria. Aside from the aforementioned effects, the selective mechanisms of the golden mussel allow it to feed on even harmful species, contributing to biomagnification and bioaccumulation processes [12]. According to Gazulha [12], the capacity to feed on toxic cyanobacteria probably allows the golden mussel to be a vector of toxins on higher trophic levels. Di Fiori [13] verified this property, which resulted in evidence that the golden mussel can accumulate and degrade the glyphosate, a poisonous herbicide used in agriculture. The ingestion of this herbicide did not directly affect the golden mussel but was shown to affect other non-target organisms in water bodies. Recent study carried out in the upper Paraná river basin (i.e. Grande River), at the Volta Grande reservoir, shows that the golden mussel also accumulates pesticides such as Endrin, p,p’-DDE, Disulfoton, Malathion and Parathion-ethyl, some of these (i.e. p,p’-DDE) with high potential of bioaccumulation [14]. A recent study by da Silva Bertão [15] reports that the golden mussel has been shown to exhibit cannibalistic behavior through filter feeding, which allows it to feed on its own larvae, microcrustaceans such as Daphniidae, Calocalanidae and protozoa beings. Sardiña [16], in an experimental study, reported that golden mussel’s larvae successfully settled among conspecifics, which may have put larvae at risk of predation within the dense aggregation of adult suspension feeders. Although there is moderate cannibalism, settlement among adult conspecifics may provide advantages to recruits such as protection (increasing survival rate) allowing for faster growth and maturation (increasing reproductive rate), thus offsetting the cost of larvae lost to cannibalism [17]. Due to its behavior as a benthic species with limited movements of escape, the golden mussel is more susceptible to predators, being one of the first animals in a food web, possibly changing the diet of several species above them [1]. Being an invasive species, it does not have any natural predator, but, with its successful invasion, there was the establishment of new predator-prey relationships between this mussel and predators [1].
Fish
Fish are the main consumers of the golden mussel due to the opportunistic habits and feed plasticity exhibited by some species and a high availability of these bivalves in the invaded environment. Cataldo [18] published a review about this topic and listed 47 fish species identified as predators of the golden mussel in South America. According to the author, in some areas (i.e. the Paraná river basin), fish that consume this mussel represent >50% of the species regularly present in commercial fisheries. Furthermore, Paolucci [19] described for the first time the consuming of golden mussel veligers by fish larvae (i.e. 11 fish taxa), and in a later review on this topic Paolucci and Thuesen [20] listed a total of 18 larval fish taxa that consumed this veligers, that indirectly leads to a higher survival of these fish larvae, and hence, increasing the number of adult fish [20] as predicted by Cataldo [18], the inventory of species that consume the golden mussel will increase according to their geographical expansion and the emergence of new studies, and currently we have already seen this increased, as showing by de Ávila-Simas [21] that described 11 species further the documented ones in 2015. According to García and Protogino [22], most of the species that consume this bivalve are siluriformes. Here, although the inventory of consumers has increased substantially since 2005, a similar pattern was observed, with siluriformes remaining the main consumers, in terms of species numbers, but with a considerable increase in the number of cichliforms and characiforms consumers. Siluriformes are fish with benthic habits that preferentially forage on the substrate where they reach its better performance due to their evolutionary morphological aspects. In this sense, among other factors (i.e. abundance of siluriformes species in invaded area, density and availability of other prey items), theoretically, the fact that golden mussels live preferentially attached to bottom substrates, the encounter rates between predator and prey may be higher in this case, then resulting in a higher probability of them be preyed upon by benthic fish. Furthermore, some fish are learning to consume this invader and evidence based on stable isotope analysis suggests that a prominent amount of the anostomids fish biomass (i.e. Leporinus friderici, Leporinus striatus and M. obtusidens) are derived from golden mussels, suggesting its importance as a complementary energy source to maintain native fish populations. As a result, changes in the energetic dynamics of receptor food webs have been observed. According to Rosa [23], the incorporation of golden mussels in the diet of L. friderici, a fish known to integrate littoral energy pathways, seems to connect this fish to pelagic energy pathways, thus changing the importance of available energy sources. On the other hand, fish predators can impact golden mussel populations by predation, as recently demonstrated by ex situ [10] and in situ experimental studies. However, although some authors have discussed the potential of these fish to act as biocontrol agents for the golden mussel, several important aspects of these predatorprey interactions still remain unknown. When the fish consumes the golden mussel or other mollusks, the shell can be crushed by teeth or remain intact in its intestine. In the first case, the mussel is dead because when the shell is crushed, it can be digested. However, in the second case, the shell can protect the mussel during digestion, impacting the predator and/or the environment: the mussel could be expelled by fish defecation, exposing the predator to infections and pathogens, and/or the mussel could be released intact into the environment, contributing to the invasion process [6]. Studies have shown that some bivalves can survive to the passage through the digestive tract of some fish species, some cases expelled whole by them [21], even more, suggesting a natural dispersion mechanism between this fish species and L. fortunei. Regarding golden mussels, studies has evaluated their survival passing through the digestive tract of fish, which verified the digestive tract of fish and detected the presence of live mussels in the final portion of the intestine, mainly of Siluriformes order, showing that this bivalve, in some cases, is also able to survive against the predation and be dispersed in the environment [21,24]. Thus, one of the most important parameters to evaluate which one is the new relation (dispersal or predation), is the shell status and live mussels in the final part of intestine.
Testudines
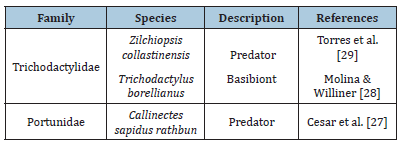
The order Testudines covers species commonly known as turtles, such as tortoise, terrapin or pond turtles and sea turtles. In South America, L. fortunei share the same environment with freshwater turtles (terrapin), which have two main families: Chelidae and Emydidae which have already been described as predators of golden mussels. The first record of a predatory relation between freshwater turtles and the golden mussel was reported by Bujes [25], who discussed the composition diet of Trachemys dorbigni, which included golden mussels. Moreover, some terrapins have the potential to disperse the L. fortunei by epibiosis, which will be discussed later in this review [25]. Cardoso [26] described the relation between four species of testudines (Table 1) and the golden mussel, considering prey and dispersion. According to this study, the predators Trachemys dorbigni and Phrynops hilarii presented high numbers of the golden mussel in its diet. Besides which, the consumption of golden mussels by these testudines was constant, indicating that feeding on these mussels was already a diet pattern. Nevertheless, the study did not elaborate on the shell status, thus it is not possible to classify these testudines as predators or dispersers at this time. An uncommon group of animals that have a new and recent relation with golden mussels are arthropods from Decapoda order. These animals are mainly omnivores and can prey or exhibit an epibiont behavior with golden mussels (Table 2). Decapoda has seven families detected in South America which two of them described as L. fortunei predators (Portunidae and Trichodactylidae). These two families of crabs have chelipeds with the function of defense and predation, being able to break the mussel’s shell or open it to expose the soft tissue, digest this and then, regurgitate the rest of valves [27,28]. One species described as a basibiont is Trichodactylus borellianus [27-30]. They are smaller than golden mussels and, in this case, the size differences are significant because a single mussel can limit T. borellianus movements, reproduction and feeding, causing an imbalance in the native population. Aside from the aforementioned characteristics, T. borellianus has the same diet as golden mussels, both feeding on protozoa, rotifers, copepods and algae which promotes competition between them [28].
Table 1:Relation of Freshwater turtles with Limnoperna fortunei in South America.
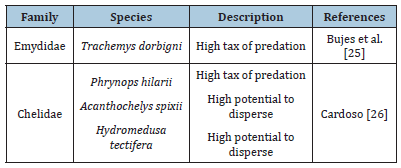
Table 2:Relation of decapods with Limnoperna fortunei in South America.
Cnidarian
Native to the Ponto-Caspian region, Cordylophora caspia, belonging to the phylum Cnidaria, class Hydrozoa, is another invasive species in Brazil, and also establishes complex relations with the L. fortunei such as mutualism, that contributing to potentialize the impacts of these two species in structures of hydroelectric power plants [15]. These two invaders were recorded together at the hydroelectric power plant of Governador Jose Richa in Paraná State, Brazil and was described that initially, C. caspia dominates the substrates and, only after some time, L. fortunei manages to settle in places previously leached by C. caspia or on the filamentous network of the cnidarian [30]. Recently, da Silva Bertão [15] observed this relation of epibiosis in which the hydrozoan serves as a substrate for the fixation of the L. fortunei, helping their larvae avoid water hydrodynamics and, preventing them from being expelled by water current. This ability to serve as microhabitats has been reported for C. caspia harboring microalgae [30]. However, larvae of L. fortunei were seen in feeding polyps of Cordylophora sp., showing a predation relationship between these invaders [15]. This feeding behavior of C. caspia was also shown with larvae of Dreissena bugensis, the quagga mussel, an invasive bivalve from North America [31]. New records of the co-occurrence between L. fortunei and C. caspia are needed to better assess these complex relationships, as in the recent study by de Paula [32] who observed the association of both these invaders at the São Simão hydroelectric power plant, state of Goiás, Brazil. Despite the few studies reporting the inclusion of different life stages of L. fortunei as part of the diet of different groups of animals, with a greater focus on fish (due to the greater interest in this relationship), animals that, despite preying, release the shell intact, they cease to be predators and become potential dispersers. Although there are no reports that the digestive tract of predators is a stressor for L. fortunei, when released with the shell intact in a new environment, this passage through the digestive tract could trigger stress signals in the golden mussel, leading to an increase in production of gametes by the mollusk, as it was observed that under stress, the golden mussel releases a greater amount of gametes into the water [2] which could increase the chance of success in an invasion event, transported in the digestive tract of fish, for example. Among the few studies that evaluate the dispersion of L. fortunei, it is noted that anthropic actions are the most relevant for this dispersion, however this balance (non)predation-(new)dispersion, can contribute to frames of new invasion events mediated by different organisms, leading to the establishment of dual ecological relationships, as seen when C. caspia co-occurs with L. fortunei.
Dispersal of Limnoperna fortunei
Blackburn [33] proposed four stages in an invasion process: transport, introduction, establishment and spread. The golden mussel managed to pass through these four stages in South America, and this success is due to many characteristics that have previously mentioned above, such as the protective shell, byssus thread, the suitable climate and be commensal with human activity (e.g., ship ballast-water transport). In this section, we will aim for the fourth stage: spread. According to Boltovskoy [34], there are two types of dispersal described for invasive species: gradual expansion and “the jump dispersal”. The first happens naturally when a population increases and spreads in an environment. The second depends on “long distance dispersal agents” which can be mediated by anthropic activities such as fishing, boating, shipping and animal traffic, or natural, such as those that happen as a consequence of storms and flooding events. Based on a study of Zhan [35], who monitored the dispersal of the golden mussels using molecular markers, it was observed that their dispersal was mainly due to anthropic activities. Its gradual expansion and establishment in an invaded environment are results of their great fecundity, resulting in approximately 30,000 eggs per female and planktonic larvae stage which can be spread through the water reaching different locations. From the first record, in Rio de la Plata estuary, the golden mussel in South America went through about 3,000 kilometers (an average of 250km per year), reaching the Paranaíba river in 2003 [34]. Considering that distance, the main mechanism of dispersal of golden mussels probably is through commercial vessels, an activity very common in the region until today and represents a great way to help it to achieve long distances [34]. Currently, there are some reports about the occurrence of golden mussels in distant locations from Rio de la Plata, such as a study of Barbosa [36] that record the presence of the golden mussel in the São Francisco River basin (dispersing the golden mussels for more than 1500 kilometers). Senske [37] reported the first register at the Santos bay-estuary complex, Saint Vincent and Bertioga channel in São Paulo and related this invasion with ballast water, the main golden mussel’s mechanism of dispersal. These new report regions far away from Rio de la Plata reflect the successful spread of it.
Ludwig [38] report a new insight on the distribution of the
golden mussel, using a molecular approach. The data obtained
revealed at least five main introduction routes in South America:
a) Japan to Rio de La Plata River.
b) China to Rio de la Plata River.
c) Rio de la Plata River to Lagoa dos Patos lake in Brazil.
d) Rio de la Plata River to Lagoa Mirim.
e) China to São Francisco River basin.
These findings demonstrated that the golden mussel possibly had at least two main invasion routes from Southeast Asia that occurred at different times, through ballast water. Comparing the haplotypes of different individuals from different populations of the golden mussel to those from São Francisco River basin it was found that the current invasive population showed more similarities to the current population in China than the population in South America since 90’s, possibly indicating multiple invasion routes, even between the basins of South America. Moreover, Belz [24] described four dispersal vectors of the golden mussel inside South America: by sand transport, through adhesion of byssus on hulls of sporting fishing boats, intake water in fishing boats and by live fish. The first occurs when trucks dredge sand from reservoirs and transport to construction sites or other reservoirs for maintenance. The study monitored 32 sand trucks on Paraná State and the results showed a low potential of this vector, because 93,7% of the sand was transported to construction sites, thus, the golden mussel cannot survive and while in transport, a high number of mussels died because of mechanical stress. Is important to highlight that the golden mussel if was not crushed, it could survive even out of the water for a few days [5,39] evaluate the tolerance of golden mussels to different temperatures when it is outside the water and conclude that this capacity of survival is very influenced by the climate condition, principally to temperature, being able to survive for up to 10 days in ideal conditions. The second and third vectors are related to fishing. In this case, 34 sport fishing boats were evaluated for the presence of the golden mussel on hull, anchor, debris and branches inside of them. The results indicated that the vectors related to fishing have a higher potential than the sand vector, and the probability of finding an adult mussel on fishing boats is also superior. In addition to this sampling, a questionnaire was made in which 20% of the thirty-four owners of these boats confirm that they are currently fishing in more than one region, thus, presenting a high potential for spreading of the golden mussel to non-invasive reservoirs [24]. The last vector is by live fish and how this process can occur has already been described above in “FISH”. An inspection of five species digestive systems was made and found live mussels only in the species P. granulosus. These results show that species have a potential to be important to dispersal, and they could be a natural vector in its native range [24]. An interesting instance which resulted in increased density of golden mussel populations described by de Amo [40], in which floodplains were the focus of studies. They analyzed the spatial distribution of golden mussels in 18 lakes in the upper Paraná River floodplain, which are adjacent to three rivers. From that, larval density was established and compared to the density in isolated rivers. It was concluded that the permanent connectivity between invaded environments, be they lakes or rivers, increased the population density of the golden mussel in comparison to isolated lakes because this high connectivity, causes a decrease in environmental variability that may favour the success of invasion by non-native species and flood phenomena create an extra channel for the periodic dispersal of these mussels [40].
The role of testudines in the dispersal of golden mussels has not been well documented, but Cardoso [26] performed experiments that started to disseminate a new perspective about how the golden mussel can deal with these organisms, principally in the dispersal aspect. The relation of epibiosis between testudines and the golden mussel occur when parts of turtle (Plastron, carapace, members and tail) are used as a substrate where the mussel can fix with byssus and hence is carried through the water or out of the water. The study monitored the behavior of four species (Table 1) and observed that Acanthochelys spixii, a species of testudines native from Rio Grande do Sul state, had the greater number of mussels adhered on its shell, compared with the other three species. A further experiment was performed when Cardoso took one individual of A. spixii with 45 mussels adhered on its shell and let it walk for 400 meters in land; at the end of the route, two individuals were still adhered, this may indicate that testudines really can transport the golden mussel in land for example, dispersing them to nearby lakes. This finding suggests that this relation could occur with other species, not only testudines in spite of they have not yet been reported.
Conclusions and Perspectives
This review synthesizes the current understanding of the dispersal mechanisms and trophic interactions of Limnoperna fortunei, highlighting the extensive ecological and economic impacts of its invasion in South America. The analysis reveals the central role of anthropogenic activities, such as shipping and recreational boating, in the spread of invasive species, alongside the need for deeper investigation into animal-mediated dispersal routes. Notably, interactions involving freshwater turtles, fish, and other predators remain underexplored and warrant comprehensive study to elucidate their potential in managing golden mussel populations. Understanding the biological and ecological traits of the golden mussel provides valuable insights for designing effective control measures. Public outreach programs and environmental education are essential for raising awareness of the risks associated with human-mediated dispersion, promoting preventative actions, and fostering sustainable practices among communities engaged in fishing and boating. Advances in research on L. fortunei can further refine strategies for monitoring and mitigating its spread, ultimately reducing its impact on ecosystems and economies, which emphasize the importance of continuous scientific updates to enhance prevention and management methods. By fostering collaboration between scientists, policymakers, and the public, the knowledge compiled in this review can inform proactive approaches to combat L. fortunei’s invasion and mitigate its consequences on biodiversity, ecosystem services, and infrastructure.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Funding Declaration
This work was supported by the Companhia Energética de Minas Gerais (CEMIG) - R&Ds Aneel GT-060.
References
- Darrigran G, Damborenea C (2009) Introduction to invasion biology, Darrigran Ga, Damborenea O. golden mussel in South America: Biology, dispersion, impact, prevention and control. Brazil p. 245.
- Cataldo D, Boltovskoy D, Hermosa JL, Canzi C (2005) Temperature-dependent rates of larval development in Limnoperna fortunei (Bivalvia: Mytilidae). Journal of Molluscan Studies 71(1): 41-46.
- Mansur MCD, Pereira D, Santos CP, Bergonci PEA, Thormann BM, et al. (2009) Colonization of artificial substrate by limnic macro invertebrates in the Jacuí river delta (RS, Brazil). Biotemas 22(1): 75.
- Nakamura FA, Almeida AC, Riera HE, Araújo JLF, Gouveia VJP, et al. (2014) Polymorphism of CaCO3 and microstructure of the shell of a Brazilian invasive mollusc (Limnoperna fortunei). Materials Research 17: 15-22.
- Andrade JTM, Cordeiro NIS, Montresor LC, Luz DMR, Viana EMF, et al. (2021) Tolerance of Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae) to aerial exposure at different temperatures. Hydrobiologia 848(12): 2993-3001.
- Oliveira CRC, Fugi R, Brancalhão KP, Agostinho AA (2010) Fish as potential controllers of invasive mollusks in a neotropical reservoir. Natureza and Conservação 8: 140-144.
- Adelino J, Heringer G, Diagne C, Courchamp F, Faria L, et al. (2021) The economic costs of biological invasions in Brazil: A first assessment. NeoBiota 67: 349-374.
- Garrido M, Chaparro O, Thompson R, Garrido O, Navarro J (2012) Particle sorting and formation and elimination of pseudofaeces in the bivalves Mulinia edulis (siphonate) and Mytilus chilensis (asiphonate). Marine Biology 159(5): 987-1000.
- Mansur MD, Santos C, Pereira D, Paz IP, Zurita ML, et al. (2012) Invasive limnic molluscs in Brazil: Biology, prevention and control.
- Rosa DM, Ward JE, Shumway SE (2018) Selective capture and ingestion of particles by suspension-feeding bivalve molluscs: a review. Journal of Shellfish Research 37(4): 727-746.
- Sylvester F, Dorado J, Boltovskoy D, Juarez A, Cataldo D (2005) Filtration rates of the invasive pest bivalve Limnoperna fortunei as a function of size and temperature. Hydrobiologia 534(1): 71-80.
- Gazulha V, Mansur M, Cybis L, Azevedo S (2012) Feeding behavior of the invasive bivalve Limnoperna fortunei (Dunker, 1857) under exposure to toxic cyanobacteria Microcystis aeruginosa. Brazilian Journal of Biology 72(1): 41-49.
- Di Fiori E, Pizarro H, Santos AM, Cataldo D (2012) Impact of the invasive mussel Limnoperna fortunei on glyphosate concentration in water. Ecotoxicology and environmental safety 81: 106-113.
- Sene AM, Rosa DM, Gutierre SMM, Pompeu PS (2021) Freshwater mollusks as proxies for assessing agrochemicals hazards in volta grande reservoir, Brazil. Revista Ambiente and Água 16(3).
- Silva Bertão AP, Leite RVV, Horodesky A, Pie MR, Zanin TL, et al. (2021) Ecological interactions between invasive and native fouling species in the reservoir of a hydroelectric plant. Hydrobiologia 848(21): 5169-5185.
- Sardiña P, Cataldo D, Boltovskoy D (2009) Effects of conspecifics on settling juveniles of the invasive golden mussel, Limnoperna fortunei. Aquatic Sciences 71(4): 479-486.
- Tamburri MN, Zimmer RK, Zimmer CA (2006) Mechanisms reconciling gregarious larval settlement with adult cannibalism. Ecol Monogr 77(2): 255-268.
- Cataldo D (2015) Trophic relationships of Limnoperna fortunei with adult fishes. Springer pp: 231-248.
- Paolucci EM, Cataldo DH, Fuentes CM, Boltovskoy D (2007) Larvae of the invasive species Limnoperna fortunei (Bivalvia) in the diet of fish larvae in the Paraná River, Argentina. Hydrobiologia 589(1): 219-233.
- Paolucci EM, Thuesen EV (2015) Trophic relationships of Limnoperna fortunei with larval fishes. Limnoperna Fortunei 211-229.
- Ávila SS, Reynalte TDA, Zaniboni FE (2019) Fish predators of the golden mussel Limnoperna fortunei in different environments in a south American subtropical river. Boletim do Instituto de Pesca 45(2).
- García ML, Protogino LC (2005) Invasive freshwater molluscs are consumed by native fishes in South America. Journal of applied Ichthyology 21(1): 34-38.
- Rosa DM, de Sene AM, Moreira MZ, Pompeu PS (2021) Non-native prey species supporting fish assemblage biomass in a Neotropical reservoir. Biological Invasions, 23(7): 2355-2370.
- Belz CE, Darrigran G, Netto OSM, Boeger WA, Ribeiro PJ (2012) Analysis of four dispersion vectors in inland waters: The case of the invading bivalves in South America. Journal of Shellfish Research 31(3): 777-784.
- Bujes C, Ely I, Verrastro L (2007) Trachemys dorbigni (Brazilian Slider), Diet. Herpetological Review 38(3): 335.
- Cardoso CDC (2014) Predators or dispersers? The relationship of the golden mussel Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae) with four species of turtles (Reptilia, Testudines) from the coastal plain of subtropical. Brazil p. 82.
- Cesar II, Armendáriz LC, Olalla N, Tablado A (2003) The blue crab, callinectes sapidus rathbun, 1896 (Decapoda, Portunidae) in the Rio de la Plata, Argentina. Crustaceana 76(3): 377-384.
- Molina FR, Williner V (2013) First record of the non-indigenous mussel Limnoperna fortunei (Bivalvia, Mytilidae) as an epibiont of the crab Trichodactylus borellianus (Decapoda, Trichodactylidae). Crustaceana 86(6): 682-692.
- Torres MV, Giri F, Williner V (2012) Size selective predation on an invasive bivalve, Limnoperna fortunei (Mytilidae), by a freshwater crab, Zilchiopsis collastinensis (Trichodactylidae). Journal of Crustacean Biology 32(5): 698-710.
- Portella KF, Joukoski A, Silva AS, Brassac NM, Belz CE (2009) Biofouling and chemical biodeterioration of portland cement mortar in hydroelectric power plant reservoir. Química Nova 32: 1047-1051.
- Pucherelli SF, Keele J, Passamaneck YJ, Beaver JR, Renicker TR (2016) Range expansion of the invasive hydroid, Cordylophora caspia (Pallas, 1771), in Colorado River reservoirs. Bioinvasions Records 5(3): 133-137.
- Paula RS, Cunha AF, Reis MDP, Souza CC, Oliveira RB, et al. (2024) Evidence of cryptic speciation in the invasive hydroid Cordylophora caspia (Pallas, 1771) (Cnidaria, Hydrozoa) supported by new records. Organisms Diversity & Evolution 24: 35-50.
- Blackburn TM, Pyšek P, Bacher S, Carlton JT, Duncan, RP, et al. (2011) A proposed unified framework for biological invasions. Trends in Ecology and Evolution 26(7): 333-339.
- Boltovskoy D, Correa N, Cataldo D, Sylvester F (2006) Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata watershed and beyond. Biological Invasions 8(4): 947-963.
- Zhan A, Perepelizin PV, Ghabooli S, Paolucci E, Sylvester F, et al. (2012) Scale‐dependent post‐establishment spread and genetic diversity in an invading mollusc in South America. Diversity and Distributions 18(10): 1042-1055.
- Barbosa NP, Silva FA, Oliveira MD, Santos NMA, Cardoso AV, et al. (2016) Limnoperna fortunei (Dunker, 1857) (Mollusca, Bivalvia, Mytilidae): First record in the São Francisco River basin, Brazil. Check List 12(1): 1-6.
- Senske W, Reigada A, Carli B, Ramires M, Rotundo M (2019) Record of bioinvasive invertebrates in Santos Bay Estuary Complex, são vicente and bertioga canal, sp, Brazil. Proceedings of the National Postgraduate Meeting 3(1).
- Ludwig S, Sari E, Paixao H, Montresor L, Araujo J, et al. (2021) High connectivity and migration potentiate the invasion of Limnoperna fortunei (Mollusca: Mytilidae) in South America. Hydrobiologia 848: 499-513.
- Montalto L (2015) Control of Limnoperna fortunei fouling by desiccation.
- Amo VE, Ernandes SJ, Moi DA, Mormul RP (2021) Hydrological connectivity drives the propagule pressure of Limnoperna fortunei (Dunker, 1857) in a tropical river-floodplain system. Hydrobiologia 848(9): 2043-2053.
© 2025 Rayan SDP. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






