- Submissions

Full Text
Biodiversity Online J
Trichodina Diaptomi Epibiont or Parasite?
Marcelo Silva Briano*, Araceli Adabache Ortiz, Alondra Encarnación Luévano, Jaime Antonio Escoto Moreno, Gerardo Guerrero Jiménez and Ángel Alcalá Pavia
Autonomous University of Aguascalientes, Mexico
*Corresponding author: Marcelo Silva Briano, Autonomous University of Aguascalientes, Mexico
Submission: July 26, 2021; Published: September 03, 2021

ISSN 2637-7082Volume1 Issue5
Abstract
Trichodina diaptomi (Ciliophora: Peritrichida: Trichodinidae); epibiont or parasite? In the state of Aguascalientes, Mexico: The ciliate Trichodina diaptomi [1], is recorded moving on the shell (carapace) of Diaptomid copepods: Mastigodiaptomus albuquerquensis [2]. The ciliate measures 50 to 60 microns in diameter, mostly parasitizes freshwater animals and fishes. However, recently there have been other species of micro crustaceans, both cladocerans (Daphnia laevis Birge 1878 and Bosmina huaronensis Delachaux 1918), other copepods Arctodiaptomus dorsalis (Marsh 1907) and Leptodiaptomus siciliodes (Lilljeborg 1889), with the same pattern of coexistence, moving on the carapace of these species.
Keywords: Cladocera; Copepoda; Freshwater; Zooplankton
Introduction
In the waterbodies of Aguascalientes State, there is a microscopic world that is completely unknown that is part of the plankton, in addition to other groups of invertebrates. However, there seems to be a protist that is transported by some species of invertebrates or even parasitizes them. This is the case of Trichodina diaptomi [1], which in the state of Aguascalientes has been found to move on the carapace of cladocerans and copepods (Figure 1-3). Trichodina is a ciliated 50 to 60μm in diameter that mostly parasitizes freshwater animals and fishes. However, it has been found as endo and ectoparasites or commensals of marine organisms such as Ctenophora [3], mainly fish [4-7]. In Mexico, it was found in Astyanax mexicanus in Cuatro Ciénegas, Coahuila [8]. The Trichodinids are endoparasites of the amphibian tractourogenital [9], as well as in Hydra sp. and in bivalve of the genus Mya sp. [3].
Figure 1: M. albuquerquensis (habitus), with Trichodina diaptomi in the dorsal part of the cephalothorax (arrows). Pond La Tomatina. Jesús María, Ags.
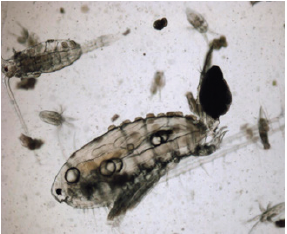
Figure 2: Zoom of Trichodina diaptomi, on the dorsal part of the cephalothorax of M. albuquerquensis. Pond La Tomatina. Jesús María, Ags.
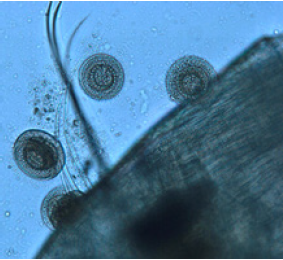
Figure 3: Arctodiaptomus dorsalis (female), habitus showing Trichodina diaptomi (arrows) sliding on the dorsal part of the cephalothorax. Bottom right, box showing zoom of the two diagnostic growths of the species located in the posterior dorsal part of the cephalothorax, showing Trichodina diaptomi. Dam Malpaso. Calvillo, Ags.

Trichodina diaptomi is distinguished by the presence of a belt or ring of calcium denticles (Figure 4&5), which provides the support to adhere to the surface (Figure 6-17) of the host [2]. At first it was thought that Trichodina diaptomi was feeding on the calanoid copepods, Mastigodiaptomus albuquerquensis and M. montezumae [2]. In addition, they present an adhesive disc that probably secretes some special glue that does not allow it to be separated from the host (Figure 18&19). After a while of observing them it was possible to appreciate that they only moved across the carapace (cephalothorax) of these crustaceans (Figure 3&4). Recently it has been found that they also thrive on other zooplankters of the cladocerans group. The aim of this study, simply to show graphically (with images) which species of zooplankton use Trichodina diaptomi as transport (foresia), to move or take them hostage for their development and survival, which inhabit the state of Aguascalientes.
Figure 4: Trichodina diaptomi, habitus showing the oral part with the calcium denticles (arrow). Pond on the side of Reservoir President Rodríguez. Jesus Maria, Ags.

Figure 5: Trichodina diaptomi, detail of the calcium denticles (arrow). Pond on the side of Reservoir President Abelardo Rodríguez. Jesús María, Ags.
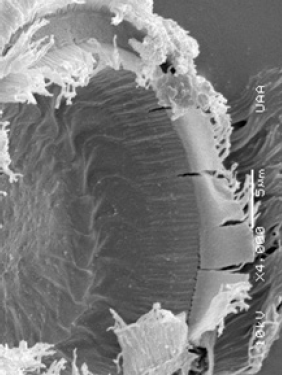
Figure 6: Trichodina diaptomi, attached to the post abdomen of Daphnia laevis (circle). Pond on the side of town Tortugas. Romos corner, Ags.

Figure 7: Trichodina diaptomi, attached to the post abdomen of Daphnia laevis (circle). Pond on the side of town Tortugas. Romos corner, Ags.

Figure 8: Trichodina diaptomi, (zoom) attached to the post abdomen of Daphnia laevis (circle). Pond on the side of town Tortugas. Romos corner, Ags.
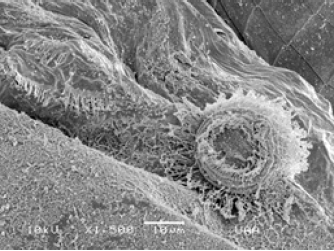
Figure 9: Trichodina diaptomi, (zoom) attached to the post abdomen of Daphnia laevis (circle). Pond on the side of town Tortugas. Romos corner, Ags.
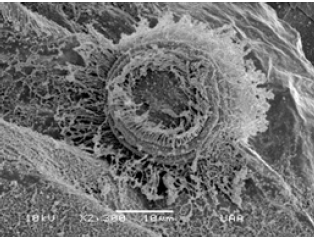
Figure 10: Trichodina diaptomi attached to the carapace of Bosmina huaroensis. El Tecuancillo Reservoir, Ags.
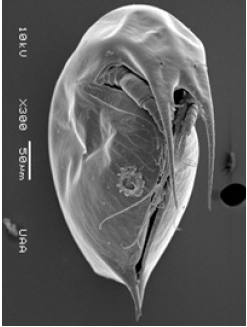
Figure 11: Trichodina diaptomi (zoom) attached to the carapace of Bosmina huaroensis. El Tecuancillo Reservoir, Ags.
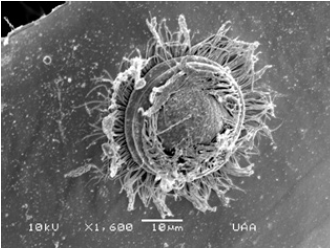
Figure 12: Leptodiaptomus siciloides, female with eggs (habitus), with Trichodina diaptomi, attached to the anterior part of the dorsal side of the cephalothorax. Puddle near town Tanque El Refugio. Asientos, Ags.
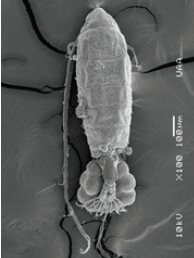
Figure 13: L. siciloides, furca of female with eggs bag, showing Trichodina diaptomi, adhered to the dorsal part of the cephalothorax. Puddle near town Tanque El Refugio. Asientos, Ags.

Figure 14: L. siciloides (female), zoom of habitus of Trichodina diaptomi, attached to the dorsal part of the cephalothorax. Puddle near town Tanque El Refugio. Asientos, Ags.
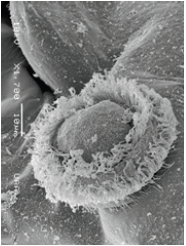
Figure 15: Zoom of the two typical growths in the posterior dorsal part of the cephalothorax in A. dorsalis, showing colonies of Colacium vesiculosum. Dam Malpaso. Calvillo, Ags.
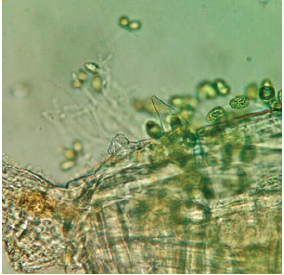
Figure 16: M. montezumae (female), habitus showing Trichodina diaptomi attached to the dorsal part of the cephalothorax. Pond on one side Reservoir President Abelardo Rodríguez. Jesús María, Ags.
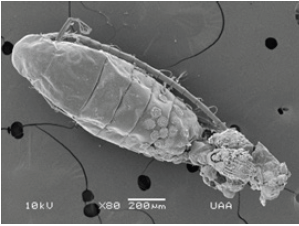
Figure 17: Zoom of Trichodina diaptomi, attached to the cephalothorax of M. montezumae. Pond on one side Reservoir President Abelardo Rodríguez. Jesús María, Ags.
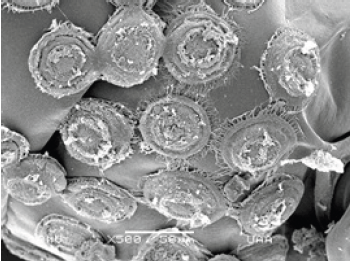
Figure 18: General view of the circle-shaped imprint left on the shell of M. montezumae by the ciliate Trichodina diaptomi. Pond on one side Reservoir President Abelardo Rodríguez. Jesús María, Ags.
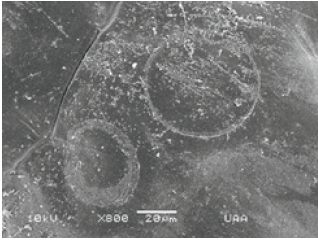
Figure 19: Zoom of the circle-shaped imprint left on the shell of M. montezumae by the ciliate Trichodina diaptomi. Pond on one side Reservoir Abelardo Rodríguez. Jesús María, Ags.
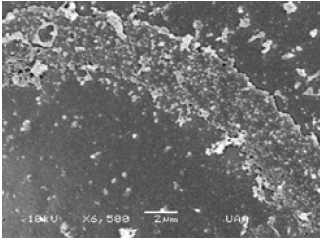
Material and Methods
The zooplankton samples were taken using a Wisconsin-type plankton net of 54 microns mesh size and fixed in 4% formaldehyde. The images were taken using the JEOL LV 5900 scanning electron microscope. Digital images were also taken with a digital camera using the PRO PLUS program, and an Apple iphone 5s.
Result
When Trichodina diaptomi was found in Aguascalientes State, there were no records in Mexico and America in general, only a reference of this ciliate in South America [1]. This work shows Trichodina using several species of zooplankton as a means of transport. Recently, there was another record of Trichodina mutabilis Kazubski and Migala 1968, in the characid Astyanax mexicanus (De Filippi 1853) of Cuatro Ciénegas, Coahuila, although not on micro crustaceans.
In general, temporary water bodies, such as a farm ponds or a small dam in the state of Aguascalientes, where the aforementioned copepods usually inhabit, there have not fish, and even the populations of these crustaceans are very large. Da Silva et al. [1] mentions that in the case of Trichodina not finding any fish in the water body, these organisms change their food habits and become bacterivores. Copepods show numerous pores on the surface of the cephalothorax (Figure 20) that definitely shows the presence of this adhesive or mucus (Figure 19) secreted by Trichodina, which suggests that there is sensitivity. At first it was thought that Trichodina diaptomi was feeding on the calanoid copepods, Mastigodiaptomus albuquerquensis and M. montezumae. After a while of observing them it was possible to appreciate that they only moved through the carapace (cephalothorax) of these crustaceans (Figure 3&4). In other groups such as the cladocerans where Trichodina has recently been found, it has not been possible to observe if the surface of the carapace shows any damage.
Following the collections of zooplankton (Rotifera, Cladocera and Copepoda), recently Trichodina diaptomi has been found in different micro crustaceans, so they all represent new records (Figure 21). The groups under study are: Cladocera and Copepoda, whose list is presented below:
Cladocera:
Daphniidae
Daphnia laevis (Figure 5-8)
Bosminidae
Bosmina huaronensis (Figure 9&10).
Copepoda:
Diaptomidae
Leptodiaptomus siciliodes (Figure 11-13)
Arctodiaptomus dorsalis (Figure 14&15)
Mastigodiaptomus albuquerquensis (Figure 3&4)
M. montezumae (Figure 16&17)
Figure 20: View of the shell of M. montezumae, where numerous pores are observed, which may indicate that there is sensitivity to Trichodina diaptomi, especially when it is fixed to this surface. Pond on one side Reservoir Abelardo Rodríguez. Jesús María, Ags.

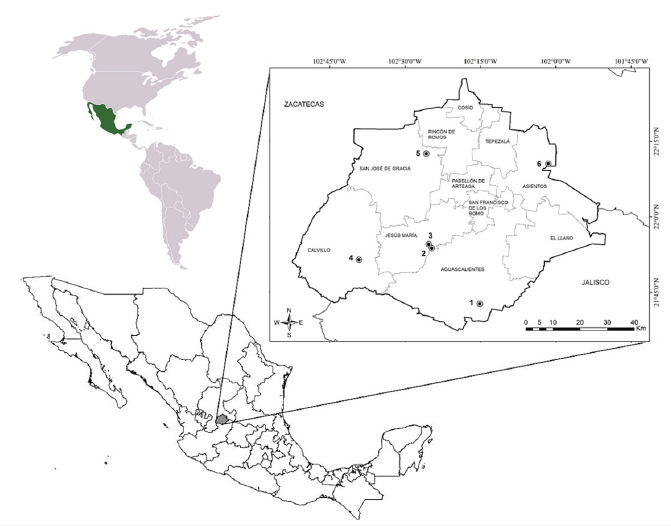
Figure 21: Aguascalientes State (Mexico). Coordinates of waterbodies where Trichodina diaptomi was found as an epibiont.1.-Reservoir El Tecuancillo, Ags: 21° 42’ 41.95’’ N; 102° 15’ 09.26’’ W; 2.-Pond La Tomatina. Jesús María, Ags: 21° 53’ 45.31’’ N; 102° 24’ 44.36’’ W; 3.-Pond on the side of Reservoir President Abelardo Rodríguez. Jesús María, Ags: 21° 54’ 32.84” N; 102° 25’ 19.62” W; 4.-Reservoir Malpaso. Calvillo, Ags: 21° 51’ 27. 12’’ N; 102° 39’ 11.95’’ W; 5.-Pond on the side of town Tortugas. San José de Gracia, Ags: 22° 12’ 32.40’’ N; 102° 25’ 48.35’’ W; 6.- Puddle near town Tanque El Refugio. Asientos, Ags: 22° 10’ 33.26’’ N; 102° 01’ 31.93’’ W.
Discussion
In general, the effects of Trichodina on fish are reported in the literature. In Mexico there are no reports of this fact, except the report of [8] where Trichodina mutabilis infects the characid Astyanax mexicanus. Another report mentions that in Brazil, Trichodina diaptomi was found in the Calanoid Notodiaptomus deitersi [1] moving along its cephalothorax (carapace) [1]. However, there are no reports about the presence of Trichodina in other zooplanktons, until today they have been registered in the state of Aguascalientes. It seems that Trichodina diaptomi has a wide variety of groups and species of micro invertebrates used as a transport, since at first it had only been reported in copepods. Cladocerans are also included in this type of interaction. As it was possible to verify, it does not produce any damage (or at least no damage was observed) to the hosts, although the trace left on the host can be observed.
It is necessary to continue with this type of research to know more about this type of ecological interactions with Trichodina diaptomi, since the implications of the species involved, and this ciliate are unknown. It is also necessary to know if they are hosts of other groups of invertebrates, since they have not yet been observed in rotifers, as with other protists that invade them such as Carchesium, Epistylis, Scyphidia, Vorticella and Colacium vesiculosum, as well as several species of algae. According to the observations made in the species reported with Trichodina in their shell, there is no damage, probably because they have not been found in large quantities as in the case of Epystilis sp., which has been stored in large quantities over the head, carapace, antennae and post-abdomen in a massive way.
Acknowledgement
Special thanks to the biologists Ramsés Alejandro Rosales García and Aldanelly Herrera Meza for their contribution of several micrographs used for this work.
References
- Da Silva W, Roche KF, De Vicente FS, Delben A (2009) First record of the peritrich Trichodina diaptomi Basson and Van As 1991 (Protozoa: Ciliophora) on a South American calanoid Notodiaptomus deitersi (Poppe, 1890) (Crustacea: Copepoda). J Eukaryot Microbiol 56(4): 385.
- Silva Briano M, Suárez Morales E, Adabache Ortiz A, Reyes Flores M (2011) Two species of Mastigodiaptomus (Copepoda: Diaptomidae), hosts of the epibiotic ciliate Trichodina diaptomi (Peritricha) in North America. J Limnol 70(2): 329-333.
- Estes AM, Reynolds BS, Moss AG (1997) Trichodina ctenophorii n. sp., a novel symbiont of ctenophores of the northern coast of the Gulf of Mexico. J Eukaryot Microbiol 44(5): 420-426.
- Ghazi SM (2004) First record of Trichodina diaptomi (Dogiel 1940), basson and van As 1991, heterodentata Duncan, 1977 and T. oligocotti (Lom 1970) (Ciliophora: Trichodinidae) from Indian fishes. Pakistan Journal of Biological Sciences 7(12): 2066-2071.
- Ghazi SM (2005) Trichodina ectoparasites (Ciliophora: Trichodinidae) of fishes in India. Research Journal of Agriculture and Biological Sciences 1: 31-37.
- Kuidong XU, Song W, Warren A (1999) Trichodinid ectoparasites (Ciliophora: Peritrichida) from the gills of cultured marine fishes in China, with the description of Trichodinella lomi n. sp. Syst Parasitol 42(3): 219-227.
- Tomas Jerônimo G, Da Costa N, Benites S, Dias Neto J, Pilarski F, et al. (2012) Trichodina colisae (Ciliophora: Trichodinidae): New parasite records for two freshwater fish species farmed in Brazil. Brazilian Journal of Veterinary Parasitology 21(4): 366-371.
- Islas Ortega A, Aguilar R (2014) Trichodina mutabilis (Protozoa: ciliates: Trichodinidae) characid fish from the Mexican tetra in the Cuatro Cienegas region, Northern Mexico. Mexican Journal of Biodiversity 85(2): 613-616.
- Fernandes MN, Sartini B, Dias RJ, Dagosto M (2011) Quantitative study of Trichodina heterodentata (Ciliophora: Mobilia) infrapopulations infesting tadpoles of a Brazilian endemic toad Rhinella pombali (Anura: Bufonidae). Zoologia 28(6): 777-783.
© 2021 Marcelo Silva Briano. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






