- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Effect of Individual vs. Combined Supplementation of Tamarind Seed Husk and Soapnut on Methane Production, Feed Fermentation and Protozoal Population in vitro
KT Poornachndra1, PK Malik1*, S Trivedi1, G Thirumalaisamy1, AP Kolte2, A Dhali2 and R Bhatta1
1Energy Metabolism Lab, Bioenergetics and Environmental Science Division, India
2Omics Laboratory, Animal Nutrition Division, India
*Corresponding author:PK Malik, Energy Metabolism Lab, Bioenergetics and Environmental Science Division, ICAR-National Institute of Animal Nutrition and Physiology, Bangalore 560030 India
Submission: July 27, 2019;Published: August 28, 2019

ISSN: 2576-9162 Volume6 Issue4
Abstract
An in vitro study was undertaken to explore the impact of individual vs. combined supplementation of tannin and saponin containing phyto-sources on total gas and methane production, feed fermentation and rumen protozoa. These phyto-sources constituted individually or in combination represented the supplementation level equivalent to 17% of concentrate or 5.1% of total diet. In this study, control treatment (T0) was formulated without any supplementation of tannin or saponin source. Among the treatments, lowest methane production was recorded in treatment T1 (3.91 ml/200 mg DM). Combined supplementation of tamarind seed husk and soapnut (T3 and T7) in this study exhibited lower methane production as compared to control (T0). On the contrary, other treatments either with individual supplementation of soap nut (T2) or combined supplementation (T4, T5 and T6) have not shown any decrease in methane production with reference to control. In general, the magnitude of methane reduction was comparatively higher in the treatments where tamarind seed husk was supplemented individually (T1, 29.8%) or in higher proportion in combined preparation with soapnut (T3, 19.4%). A decrease of 2.5 units in dry matter digestibility irrespective of the treatments was recorded due to the supplementation. A significant reduction in rumen protozoa (x105/ml) was recorded in all the test treatments and the intensity of reduction was comparatively more with individual or combined preparation where soapnut made the larger proportion. Results indicated that the reduction in protozoa always not accompany with a concurrent decrease in methane production.
keywords Environment; Methane; Mitigation; Soapnut; Tamarind seed husk
Introduction
Increasing concentration of greenhouse gas in the atmosphere is leading to the prompt shifts in climate across the globe. Intergovernmental Panel on climate change (IPCC) has listed six gases namely carbon dioxide, methane, nitrous oxide, hydrofluorocarbons, perfluorocarbons and sulphur hexafluoride [1]. Among agriculture sector, enteric fermentation is the single largest source of methane emission [2]. Livestock contribute up to 18% of global greenhouse gas emissions in the form of methane [3]. Livestock are annually emitting about 90Tg of methane due to the enteric fermentation [4]. The contribution of Indian livestock to global enteric methane emission is 9.25Tg per year [5]. Methane is listed as one of the important greenhouse gases not because of abundance, but also due to its global warming potential. Due to the massive negative environmental impacts and reduce animal feed efficiency, a search for developing suitable and effective methane mitigation strategy in ruminants is underway. Since last two decades, many strategies have been developed and tested for the reduction in enteric methane emission; however, most of them could not be implemented at the field level. The factors such as variable reduction, cost of test materials, transitory effect, impact on host animal and denizen microbes pose hindrances in the adoption of such approaches at the farmer’s doorstep [6]. For example, ionophores such as monensin and lasalocid have been largely used for the reduction of enteric methane emission; however, the results always remained inconsistent [7]. Further, their use in animal diets has been restricted in many countries (EU directive 1831/2003/Cee; [8]). Plant secondary metabolites such as tannins and saponins have shown promising results in modulating rumen microbes, rumen fermentation [9], and methane emission. Due to the natural origin of tannin or saponin, the methane emission can be controlled in a most appropriate manner by the feeding of plant secondary metabolites-containing phyto-sources at the optimum level.
Anti-methanogenic property of tanniferous tamarind (Tamarindus indica) seed husk has recently been explored both in vitro and in vivo [10]. These studies confirmed a reduction of 17-20% in enteric methane emission with the incorporation of tamarind seed husk at 5% level in finger millet straw and concentrate based diet. The impact of saponins on methane emission has also been studied [11,12]; however, the results with saponins or soap nut (Sapindus mukorossi) remain inconsistent [13] from negligible [14] to significant reduction [15,16]. Tannins primarily reduce methane emissions through a direct inhibition of the rumen methanogens [17]. On the other hand, saponins inhibit methane emissions through an indirect mechanism of reducing protozoa [10,18,19]. Currently, limited information is available in the public domain whether combined supplementation of tannins and saponins has any synergistic effect on methane reduction. In present study, we hypothesized that the combined supplementation of tamarind seed husk (tannins) and soap nut (saponins) due to their diverse mode of action would achieve more methane reduction than the individual supplementation. To test the hypothesis, an in vitro study was carried out with following objectives 1) To compare the methane production among individual and combined supplementation of tamarind seed husk and soapnut and 2) Find out the most effective tamarind seed husk and soap nut combination in terms of methane reduction for further in vivo study.
Material and Methods
Test materials
In the present study, tamarind seed husk (Tamarindus indica), an agricultural waste from starch industries and adequately available at cheaper price was collected from the Udupi district; while soap nut (Sapindus mukorossi) fruits were harvested from the Chikkamangaluru, Karnataka, India. Tamarind seed husk and soap nut were air dried in a well-ventilated shed for about 10-15days and stored in a dry and dark place. Seed were removed from dried soap nut fruits pulp before the storage.
Formulation of basal diets and chemical composition
Tamarind seed husk and/or soap nut individually or in combination made up 5.1% of the total diet and included as a concentrate ingredient with partial replacement (w/w) of wheat bran. These phyto-sources constituted 17 per cent of the concentrate mixture. The level of phyto-sources supplementation (5.1%) in present study was fixed on the basis of previous studies carried out by the group [10,20,21]. Total eight basal diets using finger millet straw (Eleusine coracana) and concentrate (70:30) were formulated with variable proportion of tamarind seed husk and soapnut. Basal diets formulated with variable proportion of tamarind seed husk and soapnut while keeping the supplementation level 5.1% of the diet were as follows: T0 (T:S, 0:0), T1 (T:S, 100:0), T2 (T:S, 0:100), T3 (T:S, 75:25), T4 (T:S, 60:40), T5 (T:S, 50:50), T6 (T:S, 40:60) and T7 (T:S, 25:75). The study was conducted in Energy Metabolism Laboratory of the Institute following the standard procedure and guidelines and the necessary approval was obtained for the use of animals as donor of microbial inoculum (CPSCEA 25/14/2017). Dried and milled basal diets were analyzed for the chemical constituents. Crude protein (N x 6.25) in basal diets was determined as per AOAC [20] with automatic digestion and distillation cum titration units (Gerhradt, Germany); while fibre fractions such as neutral detergent fiber (NDF) and acid detergent fibre (ADF) were analyzed according to Van Soest et al. [21]. All the analyses were carried out in triplicate and presented as g/kg DM.
Tannin and saponin determination
All the basal diets without incorporating phyto-sources (tamarind seed husk and soapnut) under investigation were subjected to the qualitative screening for the presence of tannin or saponin. The qualitative tests were performed as per Edeoga et al. [22]. For the quantification of tannin and saponin in respective sources, representative samples were ground to fine powder in a Cycoltec mill. Determination of total phenols (TP), total tannins (TT) and condensed tannins (CT) in the samples were carried out following the procedure described by Makkar [23]. Ultrasonic water bath was used for extracting the samples (0.2g) in 10mL of 70% aqueous acetone (twice) for 20min. The extracted samples were centrifuged at 6,000g for 10 min at 4 °C. The supernatants were combined and subjected to tannin analysis on the same day. TP and TT were assessed by a modified Folin-Ciocalteu method using poly vinyl poly pyrrolidone for separating non-tannin phenols (NTP) from tannin phenols. Condensed tannins were analysed by the butanol–HCl–iron method [24]. Hydrolysable tannins (HT) were estimated as the difference between TT and CT. TP and TT were expressed as gallic acid equivalents while CT as leucocyanidin equivalents. All the analyses were carried out in duplicate except for detergent fractions, which were measured in triplicate. Quantitative determination of saponin in soapnut was carried out by the method of Ejikeme et al. [25] following the repeated extraction with aqueous ethanol and n-butanol. The saponin content was expressed as a percentage.
Incubation and gas analysis
Rumen liquor was collected from three cannulated crossbred male cattle before morning feeding. The cannulated animals were fed on a diet formulated with finger millet (Elusine coracana) straw and concentrate mixture (70:30) to satisfy the maintenance requirement as per ICAR feeding standard [26]. Rumen liquor was sub-fractioned into two and the liquid phase was collected in a pre-warm (39 °C) carbon dioxide flushed thermos flask; whilst solid phase was stored in plastic bags covering with pre-warmed thermal packs. For the incubation purpose, both the sub-fractions of rumen inoculum were mixed in 1:1 and filtered through four layers of muslin cloth, pooled and finally used as inoculum for the in vitro studies. In vitro gas production technique of Menke & Steingass [27] was employed for the evaluation of basal diets comprising tamarind seed husk and soap nut in variable proportion at fixed level of 5.1% for the impact on methane production, feed fermentation and rumen protozoa. Briefly, 200mg dried basal diet sample was incubated with 30ml buffered rumen inoculum in a 100ml calibrated glass syringes (Haeberle, Germany). Initial position of piston was recorded before incubating the samples. All the basal diets were incubated in triplicate with two repeated incubations on successive weeks. Incubations without tannin and saponin phyto-sources served as negative control; while incubations without basal diet served as blank. Syringes (without basal diet) served as blank were kept in triplicate with every set of incubations. Further, Hohenheim hay served as standard reference was also incubated along with basal diet in each run [28]. Basal diets were incubation at 39 °C for 24h in a water bath shaker having provision for intermittent shaking.
On the termination of incubation, final position of piston was recorded, and total gas produced was calculated by difference. Further, a correction was also made for the total gas produced in blank syringe that contain only the inoculum and buffer. For methane analysis, 1ml gas sample was carefully transferred in an airtight glass syringe (Hamilton, USA) and injected in a gas chromatograph (7890B Agilent) fitted with packed column Porapak Q and thermal conductivity detector. Temperature of the injector oven, column oven and detector were 60, 100 and 110 °C, respectively [5]. Before analyzing the actual, a standard gas (Chemix specialties gases, Bangalore,) sample of known CH4 concentration was injected three times and recorded the peak area. The CH4 peak was identified from its retention time and calculated as follows
Based on the percentage, CH4 produced was calculated as
Dry matter digestibility, fermentation parameters and rumen protozoa
In vitro dry matter digestibility (IVDMD) of the basal diets was determined as per the method of Goering & Van Soest [29]. About 500mg dried ground sample was incubated with 40ml rumen fluid-buffered medium in 100 ml glass syringes in triplicate and incubated in a water bath at 39 °C for 24h. At the completion, the fermentation was terminated with the addition of ice cubes in water bath shaker and the whole content of syringe was poured into a fibre bag. The syringe was repeatedly washed to remove remaining residual feed and poured into the fibre bag. The NDF content in the feedstuff was estimated according to AOAC (2012; Method No. 2002:04) by Fibretherm apparatus (Gerhardt, Germany). The following equation was used to determine IVDMD
After completion of the incubation (24h), the fermentation fluid was divided into two equal parts and stored at -20 °C until further analysis. Metaphosphoric acid (25%) in 1:5 (v/v) was added to the first fraction of the fluid and preserved for total volatile fatty acid estimation; while few drops of saturated HgCl2 were added to another sub-set of fluid and stored for ammonia-N determination. Fluid samples were taken out from -20 °C, thawed at room temperature and analyzed for ammonia-N [30] and total volatile fatty acid [31]. Rumen protozoa were identified according to the Hungate [32]. Briefly, samples were mixed with methyl greenformalin- saline solution (1:1) and mixed thoroughly. Two drops of this sample were placed in Neubauer’s hemocytometers and did the enumeration as described by Kamra et al. [33].
Statistical analysis
Data were analyzed to explore the effect of tannin and saponin phyto-source supplementation on in vitro methane production, feed fermentation characteristics and rumen protozoa in following model using MINITAB17
Where, Yijsub> represented individual observation, μ was the general mean, Ai was the effect of the treatment (i= 1-15) and εij was the experimental error. Superscripts were placed wherever mean values among the treatments proved significant.
Results and Discussion
Results from the studies are presented and discussed under various heads in this section.
Chemical composition of phyto-sources
Data pertaining to the chemical composition have been presented in Table 1. Crude protein (CP%) content varied between 9.38-10.2% among different basal diets formulated with variable proportion of tamarind seed husk and soap nut. The CP content (%) in tamarind seed husk in present study was comparable with the previous findings of Bhatta et al. [9,34]. On the other hand, Malik et al. [10] reported comparatively higher crude protein in tamarind seed husk than the present study. This deviation in crude protein could be due to the difference in samples, harvesting stage and processing of the husk. In present study, an average 2.78% CP was reported in soap nut fruit pulp and due to the inadequacy of data, the CP in soap nut could not be compared with others. There was no substantial difference in neutral detergent fibre (NDF) and ADF (acid detergent fibre) was reported among the basal diets (Table 1). The fibre fractions represented by NDF & ADF in the present study were in consonance with Bhatta & Malik [9,10]. The information on the fibre fractions of soap nut was also not available and hence could not be compared with others. Further, the organic matter and total ash were comparable among the treatments. Out of total eight, six basal diets contained condensed tannins and saponins at variable levels between 1.78-7.14 and 1.29- 5.15 g/kg DM, respectively. This variation in tannin and saponin concentration among the basal diets was obviously due to the variable proportions of tamarind seed husk and soap nut in finger millet straw and concentrate based diet at fixed level of 5.1% (Table 1).
Table 1:Chemical composition (g/kg DM) of basal diets.
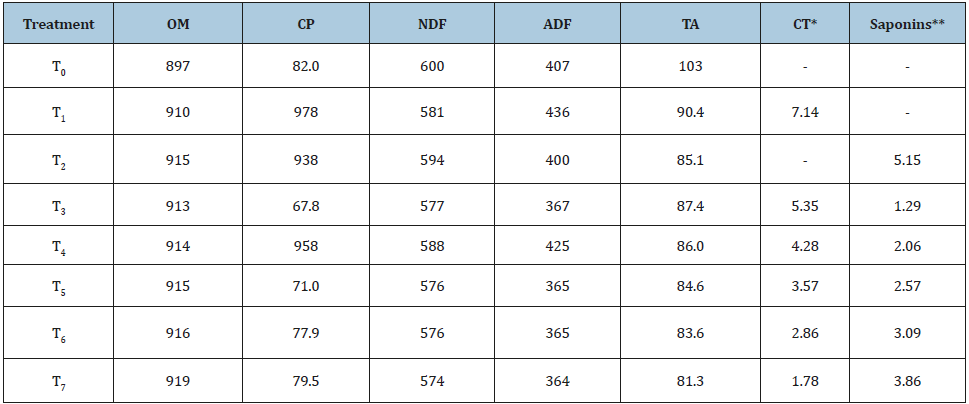
T0 (control, no supplementation), T1 (tamarind seed husk supp.), T2 (soapnut supp.), T3 (tamarind seed husk and soapnut supp. 75:25), T4 (tamarind seed husk and soapnut supp. 60:40), T5 (tamarind seed husk and soapnut supp. 50:50), T6 (tamarind seed husk and soapnut supp. 40:60), T7 (tamarind seed husk and soapnut supp. 25:75). (OM: organic matter; CP: crude protein (N x 6.25); NDF: neutral detergent fiber; ADF: acid detergent fiber; TA: total ash; *CT: condensed tannin; CT was measured as leucocyanidin equivalent and presented as g/kg DM
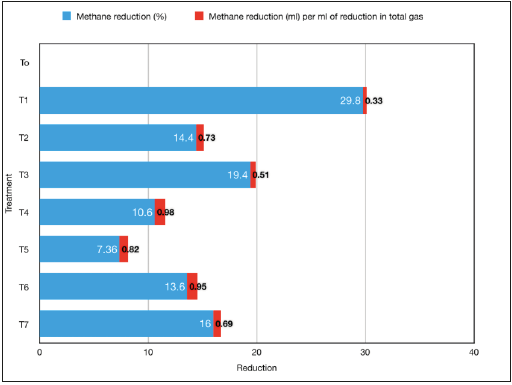
Effect on in vitro total gas and methane production Total gas production (ml/200mg substrate) as compared to control (T0) was adversely affected (p<0.05) with the individual supplementation (T1) of tannin-containing tamarind seed husk at 51g/kg level yielding condensed tannin equivalent to 7.14 g/ kg of diet. Total gas production was not affected (p>0.05) by the individual supplementation of soap nut (T2) or combined supplementation of tamarind seed husk and soap nut at variable proportion (T3-T7). The differential response of phyto-sources at the similar level of supplementation (5.1% of diet) in present study was due to the difference in plant secondary metabolite concentration. The concentration of condensed tannin in tamarind seed husk was 140g/kg DM; whilst saponin content of the soap nut was 101g/kg DM. Thus, at similar level (5.1%) the individual supplementation (100:0) of tamarind seed husk (T1) decreased impact on decreasing total gas production as compared to soap nut (T2). These results are in good agreement with the recent findings of Malik et al. [10], reported that the reduction in total gas production was associated with the level of tamarind seed husk in the diet. They have not reported any adverse impact of tamarind seed husk supplementation on gas production at 2.5% supplementation level; whereas the gas production was significantly decreased as the levels increased to 5.0 and 10.0%. From the results it can be inferred that the reduction in gas production was dose dependent and affected with the concentration of tannin and saponins concentration supplied through phyto-sources at specified level of inclusion. Methane production as influenced with the supplementation of tannin and saponin containing phyto-sources either individually or in variable proportions of both is presented in Table 2. Among the treatments, lowest methane production was recorded in treatment T1 (3.91ml/200mg DM) where tamarind seed husk was supplemented alone as a condensed tannin source at 5.1% of the basal diet (Table 2). Similarly, methane production in treatments T3 and T7 was also significantly lower (p<0.05) as compared to control (T0). However, the variation in methane production among other test groups (T2, T4, T5 and T6) did not differ from the methane in control. In present in vitro study, a wide range of reduction (7.4-29.8%) in methane production due to the individual or combined supplementation of tamarind seed husk and soap nut was reported. In general, the magnitude of methane reduction was comparatively higher in the treatments where tamarind seed husk was supplemented individually (T1, 29.8%) or in higher proportion in combined formulation (T3, 19.4%). Incorporation of plant secondary metabolites containing phyto-sources in the diet lead to a reduction in methane via the inhibition of rumen methanogens [35-37], reduction in rumen protozoa [18,38,39], depression in fibre digestion [10,40] and shift in the fermentation towards more propionate production [41-43]. Comparison of methane production among various treatments in present study would fail to draw any meaningful inference until the reduction in methane is equated with the reduction in total gas. Results from the study indicated that the individual supplementation of tamarind seed husk in treatment T1 lead to a moderate reduction of 0.33ml methane per ml of reduction in total gas (Figure 1). On the other hand, combined supplementation of tamarind seed husk and soap nut in 60:40 ratio (T4) induced highest reduction in methane production (0.98ml/ml reduction in total gas). From these results, it is evident that the combined supplementation of tamarind seed husk and soap nut in a specific ratio (60:40) had more pronounce effect on methane production than the individual supplementation without compromising the feed fermentation.
Table 2:Effect of tannin and saponin combined supplementation on in vitro gas production.
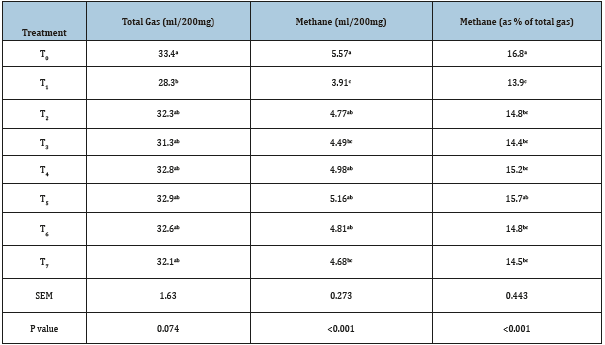
Mean values bearing different superscripts in a column differ significantly (p<0.05); T0 (control, no supplementation), T1 (tamarind seed husk supp.), T2 (soapnut supp.), T3 (tamarind seed husk and soapnut supp. 75:25), T4 (tamarind seed husk and soapnut supp. 60:40), T5 (tamarind seed husk and soapnut supp. 50:50), T6 (tamarind seed husk and soapnut supp. 40:60), T7 (tamarind seed husk and soapnut supp. 25:75).
Figure 1:Effect of tannin and saponin supplementation (individual vs. combined) on in vitro methane reduction. T0 (control, no supplementation), T1 (tamarind seed husk supp.), T2 (soapnut supp.), T3 (tamarind seed husk and soapnut supp. 75:25), T4 (tamarind seed husk and soapnut supp. 60:40), T5 (tamarind seed husk and soapnut supp. 50:50), T6 (tamarind seed husk and soapnut supp. 40:60), T7 (tamarind seed husk and soapnut supp. 25:75).
In a recent study, [10] tamarind seed husk supplementation at 2.5% has been found ineffective in reducing the methane emission; whilst increasing level of supplementation to 5.0% decreased (p<0.05) enteric methane emission in cattle. On the contrary, the inclusion of tamarind seed husk at almost similar level (3.0%) as that in study of Malik et al. (2017a) with soap nut in 60:40 achieved the highest reduction in methane production. These findings imply that there was an interaction between tannin and saponin and associative action of both led to a decrease (p<0.05) in methane production. Our findings are in consonance with Poornachandra et al. [21], who reported that the combined supplementation of tamarind seed husk and soap nut at 5.1% in 60:40 achieved significant reduction in enteric methane emission and was found equally effective as that of the individual supplementation of tamarind seed husk at similar level. In contrast, soap nut supplementation at similar level in a short duration study in cattle was not found effective in reducing methane emission [21]. These results are in agreement with previously reported studies [44-46], wherein it is established that the combined supplementation of tannin and saponin had associative effect on decreasing methane. Makkar et al. [47] reported that the interaction between tannin and saponin led to the additional reduction in methane production. Maximum reduction in methane production with combined supplementation of tamarind seed husk and soap nut in 60:40 was attributed to diverge and associative action of both the plant secondary metabolites. Anti-methanogenic action of tannin [1,10,48,49] and defaunating effect of saponin are documented [50,51]. Though the methanogens density among different treatments in present study was not estimated, nevertheless it is reported that tamarind seed husk decreases rumen methanogens numbers and thereby decrease methane emission [10,20,21]. An established mechanism of toxicity to methanogens is the tanning of protein located at accessible sites in methanogens [35,36]. Saponins have been known for antimethanogenic properties due to their selective inhibitive action on protozoal cell membrane integrity [50]. The presence of cholesterol and other sterols in protozoal cell membrane make them vulnerable for the selective inhibition by saponin, which has affinity towards cholesterol. Thus, the additional reduction in methane production with combined supplementation of tamarind seed husk and soap nut (60:40) as compared to their individual supplementation could be due to the cumulative action of both methanogens and protozoa reduction. In addition, a decrease in dry matter digestibility may have contributed to the less methane production in individual and combined supplementation treatments. However, the adversity of tamarind seed husk and soap nut when supplemented together in 60:40 was not apparent in an in vivo study [20]. Similarly, individual supplementation of tamarind seed husk and soap nut in cattle at similar level did not produce any adverse impact on the dry matter digestibility and intake. This deviation in results called for a caution that in vitro results should be carefully extrapolated with in vivo. The variation in dry matter digestibility at similar level between in vitro (present study) and in vivo [21] experiments was certainly due to the factors such as microbial adaptation, palatability of test material, continuous media flow and direct absorption [52].
IVDMD and fermentation characteristics
In this study, an adverse impact of tamarind seed husk and soap nut supplementation on the dry matter digestibility (Table 3) was recorded. As compared to control, an average decrease of 2.5 units with the individual or combined supplementation of tamarind seed husk and soap nut at variable proportion was recorded. Results indicated that the individual supplementation of soap nut at 5.1% level comparatively led to a higher decrease in dry matter digestibility than the tamarind seed husk (2.3 vs. 2.7 units). Similarly, the increasing levels of soap nut in the combined supplementation led to higher decrease in dry matter digestibility (Table 3). Malik et al. [53] did not find any reduction in dry matter digestibility due to the inclusion of tropical tree leaves supplying 7.15-10.8 g/kg DM tannin in sheep. However, the adverse impact of plant secondary metabolites was evident in present study at a lower level of tannin (7.14g/kg DM) and saponin (3.86g/ kg DM). Findings of Gerlach et al. [54] are in good agreement with our results, reported a decrease in dry matter digestibility with almost similar tannin concentration (2.3-10.2g/kg DM). Recently, Poornachandra et al. [21] have not reported any impact of the individual or combined (60:40) supplementation of tamarind seed husk or soap nut at similar level (5.1%). On the contrary, Malik et al. [10] reported an adverse impact of tamarind seed husk supplementation (10% of diet) equivalent to ~15g condensed tannin per kg of DM on dry matter digestibility. This variable impact of tannins to exert adverse impact with lower doses than general recommendation (<20g/kg DMI) can be justified on the basis of structural differences among tannins [54]. One of the reasons for decrease in dry matter digestibility with the individual and combined supplementation could be the type of secondary metabolites and concentration in the phytosource. This deviation in results can be attributed to the phytosources and type of study. Jayanegara et al. [52] and Poornachandra et al. [21] included that in vitro results due to variable response of microbes, microbial adaptation, palatability of test material, continuous media flow, direct absorption and continuous Vs. static flow of secondary metabolites in the medium vary from the in vivo studies. Impact of plant secondary metabolites on dry matter digestibility per se is dose dependent [55]. In our study, total VFA production was decreased significantly (p<0.05) with the individual supplementation of tamarind seed husk (T1), soap nut (T2) or combined supplementation of both in variable proportion, but at the similar level of 5.1% (Table 3). The magnitude of decrease in TVFA production in test treatments was 0.27 (T1) to 0.55 units (T2). The slight reduction in total volatile fatty acid production due to the individual or combined supplementation of tamarind seed husk or soap nut can be explained on the basis of reduction in dry matter digestibility. These results indicated that the TVFA production depends on the feed fermentation as highest reduction in dry matter digestibility (T2) in present study led to maximum decrease of 0.55 units (T2) in TVFA production as compared to control. On the other hand, Poornachandra et al. [21] reported a significant increase in TVFA production due to the supplementation of soap nut or tamarind seed husk and soap nut combo (60:40) at a level of 5.1% of the basal diet in crossbred cattle. This ambiguity in the results obviously attributed to the dry matter digestibility between two studies
Table 3:Effect of individual vs. combined supplementation on digestibility and feed fermentation.
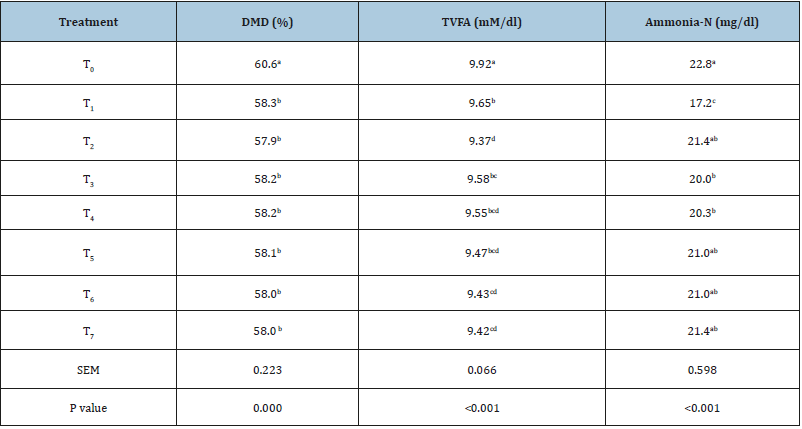
Mean values bearing different superscripts in a column differ significantly (p<0.05), IVDMD: in vitro dry matter digestibility; TVFA: total volatile fatty acid; Ammonia-N: ammonia nitrogen; T0 (control, no supplementation), T1 (tamarind seed husk supp.), T2 (soapnut supp.), T3 (tamarind seed husk and soapnut supp. 75:25), T4 (tamarind seed husk and soapnut supp. 60:40), T5 (tamarind seed husk and soapnut supp. 50:50), T6 (tamarind seed husk and soapnut supp. 40:60), T7 (tamarind seed husk and soapnut supp. 25:75).
Inhibitory effect of tamarind seed husk on the protein degradation was apparent with a significant decrease in ammonia concentration in present study and individual supplementation of tamarind seed husk (T1) or larger proportion in combined formulation (T3) led to a decrease (p<0.05) in ammonia concentration as compared to control (Table 3). However, the individual soap nut (T2) or combined supplementation where larger proportion was represented by soap nut (T2, T5-T7) did not exhibit any significant (p>0.05) reduction in ammonia concentration. Findings of Poornachandra et al. [21] strengthen our results, reported a significant decrease in ammonia concentration due to the incorporation of tamarind seed husk alone or combined with soap nut in 60:40 at the similar level of 5.1% of the diet. Decrease (p<0.05) in ammonia concentration with tamarind seed husk or treatments with larger proportion of tamarind seed husk could be an artifact of the tannin-protein complexes that resulted into less protein degradation.
Effect on protozoa
A significant reduction (p<0.05) in total protozoa (x 105/ml) was recorded in all the test treatments as compared to control. The intensity of reduction in total protozoa was more with individual or combined preparation where soap nut made the larger proportion of the supplementary level 5.1%. Taking all together, soap nut was found more effective than the tamarind seed husk in reducing the rumen protozoal numbers (Table 4). Entodinomorphs irrespective of the dietary treatments represented the largest fraction of the rumen protozoa and were decreased (x 105/ml) more intensively with soap nut supplementation as compared to tamarind seed husk. Malik et al. [10] in an in vivo study reported a significant (p<0.05) reduction in total protozoal population due to the graded supplementation of tamarind seed husk in finger millet and concentrate-based diet at 2.5 and 5.0% levels. Our findings are in good agreement with earlier reports [10,39,53,56,57] revealed that entodinomorphs were major protozoa in the rumen. Recently, Poornachandra et al. [21] also reported a significant reduction (p<0.05) in total rumen protozoa, entodinomorphs & holotrich with the individual supplementation of similar sources i.e. tamarind seed husk and soap nut and combined supplementation of both in 60:40. Saponin forms irreversible complexes with cholesterol and caused the lysis of cell membrane [58] and thereby reduce protozoal numbers [47]. A decrease in protozoal numbers due to their ecto or endosymbiotic relationship with methanogens may be accountable for the reduction in methane emission. Recently, from an in vivo study Poornachandra et al. [21] concluded that in spite of higher protozoal number reduction with saponin supplementation (5% of diet) in crossbred cattle, there was no significant reduction in rumen methanogenesis. In line with our results, the supplementation of tamarind seed husk or combo of tamarind seed husk and soap nut (60:40) in spite of less protozoal reductionled to comparatively more methane decrease than the individual supplementation [19]. These results indicated that the reduction in protozoa always not accompanied with a concurrent decrease in methane as only 9-25% of the rumen methanogens are associated with protozoa [35,59]. It is quite possible that soap nut saponin perhaps not targeted archaea-associated protozoa and therefore, no significant reduction in methane production was reported with individual supplementation of Soapnut [60,61].
Table 4:EEffect of individual Vs combined supplementation of tannin and saponin sources on rumen protozoa (X 105/ ml).
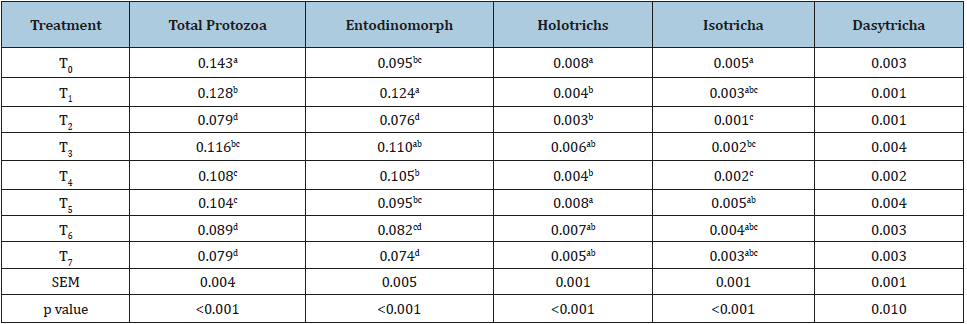
Mean values bearing different superscripts in a column differ significantly (p<0.05), T0 (control, no supplementation), T1 (tamarind seed husk supp.), T2 (soapnut supp.), T3 (tamarind seed husk and soapnut supp. 75:25), T4 (tamarind seed husk and soapnut supp. 60:40), T5 (tamarind seed husk and soapnut supp. 50:50), T6 (tamarind seed husk and soapnut supp. 40:60), T7 (tamarind seed husk and soapnut supp. 25:75).
Conclusion
The study concludes that the individual supplementation of tamarind seed husk at 5.1% level and equated to 7.0g/kg DM tannin achieved a reduction of 29% in methane production. However, individual supplementation of soap nut at similar level in spite of the substantial protozoal decrease did not cause any significant reduction. Combined supplementation of tamarind seed husk and soap nut (60:40) at similar level (5.1%) found effective than the individual supplementation of tamarind seed husk or soap nut considering the reduction in total gas production [62,63]. These results provide an insight, however, in vivo studies in ruminants with individual and combined supplementation of selected sources are mandatory to confirm the methane reduction and its extent along with underlying mechanism accountable for the decrease.
Economic Consequence: Farmer’s perspective
Under present scenario of quality feed shortage and intensification of livestock productivity, farmers are extremely pressurized. Amelioration of livestock methane in natural ways through feeding approaches in one of the biggest challenge. In present study, tamarind seed husk used as a source of condensed tannin is an agricultural waste from the starch industry and adequately available. Similarly, soap nut is also natural agricultural produce, does not compete with human food and also not very expensive. Since both tamarind seed husk and soap nut are agricultural produce, not expensive therefore, a combined formulation as proposed in this study can be ideal candidates for the effective control of enteric methane emission from ruminants.
Way Forward
Though individual supplementation of tamarind seed husk in the present study proved a potential methane suppressant, however, the level used for individual supplementation (5.1%) may negatively affect the feed intake and fermentation. On the contrary, combined supplementation of tamarind seed husk and soap nut where 3.1% constituted by tamarind seed husk and rest 40% by the soap nut is quite safe and recommended over the individual supplementation at similar level of 5.1%. Findings from the study indicated that there could be a synergistic effect of soap nut in the presence of tamarind seed husk. Further, short and long-term studies are warranted in ruminants with individual and combined supplementation of selected sources.
Acknowledgement
The authors are grateful to the Department of Biotechnology (DBT), Govt. of India, New Delhi for providing the financial support to carry out this research work under the project (BT/PR8750/ AAQ/1/555/2013) entitled “Livestock methane reduction through immunization based approach”. The first author is also thankful to the Director, National Institute of Animal Nutrition and Physiology, Bangalore for extending facilities for the study.
References
- Bhatta R, Saravanan M, Baruah L, Prasad CS (2015) Effects of graded levels of tannin-containing tropical tree leaves on in vitro rumen fermentation, total protozoa and methane production. J Appl Microbiol 118(3): 557-564.
- Shibata M, Terada F (2010) Factors affecting methane production and mitigation in ruminants. Anim Sci J 81(1): 2-10.
- Mara OFP (2011) The significance of livestock as a contributor to global greenhouse gas emissions today and in the near future. Anim Feed Sci Technol 166-167: 7-15.
- Malik PK, Singhal KK, Deshpande SB (2012) Mitigation strategies for enteric methane emission with special emphasis on biological approaches: A review. Indian J Anim Sci 82: 794-804.
- Bhatta R, Malik PK, Kolte AP (2019) Assessment of enteric methane emission from Indian livestock: A new approach. In: 7th Pan Commonwealth Veterinary Conference on the role of veterinarians in addressing the global challenges to the lives of our pets, livestock, wildlife, humans and environment held at NIANP, Bengaluru from 3-7th March 2019.
- Malik PK, Bhatta R, Gagen EJ, Veerasamy S, Soren NM, et al. (2015a) Alternate H2 sinks for reducing rumen methanogenesis. In: Climate change impact on livestock: Adaptation and mitigation. Springer, New Delhi, India, pp. 303-320.
- Bell N, Wickersham T, Sharma V, Callaway T (2015) Ionophores: A tool for improving ruminant production and reducing environmental impact. In: Malik PK, Bhatta R, Takahashi J, Kohn RA (Eds.), Livestock production and climate change. CABI, Wallingford, pp. 263-272.
- Cheli F, Gallo R, Battaglia D, Dell Orto V (2013) EU legislation on feed related issues: An update. Ital J Anim Sci 12(2): 295-312.
- Bhatta R, Krishnamoorthy U, Mohammed F (2000) Effect of feeding tamarind (Tamarindus indica) seed husk as a source of tannin on dry matter intake, digestibility of nutrients and production performance of crossbred dairy cows in mid-lactation. Anim Feed Sci Technol 83(1): 67-74.
- Malik PK, Kolte AP, Bakshi B, Baruah L, Dhali A, et al. (2017) Effect of tamarind seed husk supplementation on ruminal methanogenesis, methanogen diversity and fermentation characteristics. Carbon Management 8(4): 319-329.
- Lila ZA, Mohammed N, Kanda S, Kamada T, Itabashi H (2003) Effect of sarsaponin on ruminal fermentation with particular reference to methane production in vitro. J Dairy Sci 86: 3330-3336.
- Malik PK, Singhal KK (2008c) Influence of supplementation of wheat straw based total mixed ration with saponins on total gas and methane production in vitro. Indian J Anim Sci 78: 298-301.
- Jayanegara A, Wina E, Takahashi J (2014) Meta-analysis on methane mitigating properties of saponin-rich sources in the Rumen: Influence of addition levels and plant sources. Asian Australasian J Anim Sci 27(10): 1426-1435.
- Mc Sweeney C, Ramírez Restrepo CA (2015) Supplementation with tea saponins and statins to reduce methane emissions from ruminants. North Sydney NSW, Sydney.
- Agarwal N, Kamra DN, Chaudhary LC, Patra AK (2006) Effect of sapindus mukorossi extracts on in vitro methanogenesis and fermentation characteristics in buffalo rumen liquor. J Appl Anim Res 30: 1-4.
- Malik PK, Singhal KK (2009) Effect of lucerne (Medicago sativa) fodder supplementation on nutrient utilization and enteric methane emission in male buffalo calves fed on wheat straw based total mixed ration. Indian J Anim Sci 79(4): 416-421.
- Bhatta R, Uyeno Y, Tajima K, Takenaka A, Yabumoto Y, et al. (2009) Difference in the nature of tannins on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J Dairy Sci 92(11): 5512-5522.
- Finlay BJ, Esteban G, Clarke KJ (1994) Some rumen ciliates have endosymbiotic methanogens. FEMS Microbiol Lett 117(2): 157-161.
- Hess HD, Kreuzer M, Díaz TE, Lascano CE, Carulla JE, et al. (2003) Saponin rich tropical fruits affect fermentation and methanogenesis in faunated and defaunated rumen fluid. Anim Feed Sci Technol 109(1-4): 79-94.
- George WL (2012) Official methods of analysis of AOAC International. (19th edn), AOAC International, Gaithersburg, Maryland, USA.
- Poornachandra KT (2017) Assessment of the combined effect of selected tanniniferous and saponin containing phyto-sources on enteric methane emission in crossbred cattle. MVSc thesis submitted to National Dairy Research Institute, Karnal, India.
- Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. African J Biotechnol 4(7): 685-688.
- Makkar HPS (2003) Quantification of tannins in tree and shrub foliage: A Laboratory Manual. (1st edn), Springer, Netherlands.
- Van Soest PJ, Robertson JB, Lewis BA (1991) Methods for dietary fiber, neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 74(10): 3583-3597.
- Ejikeme CM, Ezeonu CS, Eboatu AN (2014) Determination of physical and phytochemical constituents of some tropical timbers indigenous to niger delta area of Nigeria. Eur Sci J 10(18): 1857-7881.
- Poornachandra KT, Malik PK, Dhali A, Kolte AP, Bhatta R, et al. (2019) Effect of combined supplementation of tamarind seed husk and soapnut on enteric methane emission in crossbred cattle. Carbon Management.
- Menke KH, Steingass H (1988) Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim Res Dev 28: 7-55.
- Porter LJ, Hrstich LN, Chan BG (1986) The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 25(1): 223-230.
- Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus reagents, procedures and some applications). Agricultural Research Service/United States Department of Agriculture, Washington DC, USA, p. 20.
- ICAR (2013) Nutrient Requirements of Cattle and Buffalo. (3rd edn), Indian Council of Agricultural Research, New Delhi, India.
- Menke KH, Raab L, Salewski A, Steingass H, Fritz D, et al. (1979) The estimation of the digestibility and metabolizable energy content of ruminant feeding stuffs from the gas production when they are incubated with rumen liquor in vitro. J Agric Sci 93(1): 217-222.
- Hungate RE (1966) The rumen and its microbes. Academic press, New York, USA.
- Kamra DN, Sawal RK, Pathak NN, Kewalramani N, Agarwal N (1991) Diurnal variation in ciliate protozoa in the rumen of black buck (Antilope cervicapra) fed green forage. Lett Appl Microbiol 13(3): 165-167.
- Bhatta R, Krishnamoorthy U, Mohammed F (2001) Effect of tamarind (Tamarindus indica) seed husk tannins on in vitro rumen fermentation. Anim Feed Sci Tech 90(3-4): 143-152.
- Conway EJ (1957) Micro-diffusion analysis and volumetric error. (4th edn), Crosby Lockwood & Son ltd, Glasgow, Scotland, London.
- Barnett AJG, Reid RL (1957) Studies on the production of volatile fatty acids from grass by rumen liquor in an artificial rumen: I. The volatile acid production from fresh grass. J Agric Sci 48(3): 315-321.
- Field JA, Lettinga G (1987) The methanogenic toxicity and anaerobic degradability of a hydrolysable tannin. Water Res 21(3): 367-374.
- Mc Sweeney CS, Palmer B, Bunch R, Krause DO (2001) Effect of the tropical forage calliandra on microbial protein synthesis and ecology in the rumen. J Appl Microbiol 90(1): 78-88.
- Tavendale MH, Meagher LP, Pacheco D, Walker N, Attwood GT, et al. (2005) Methane production from in vitro rumen incubations with and effects of extractable condensed tannin fractions on methanogenesis. Anim Feed Sci Technol 123-124: 403-419.
- Newbold CJ, Lassalas B, Jouany JP (1995) The importance of methanogens associated with ciliate protozoa in ruminal methane production in vitro. Lett Appl Microbiol 21(4): 230-234.
- Tan HY, Sieo CC, Abdullah N, Liang JB, Huang XD, et al. (2011) Effects of condensed tannins from Leucaena on methane production, rumen fermentation and populations of methanogens and protozoa in vitro. Anim Feed Sci Technol 169(3-4): 185-193.
- Malik PK, Singhal KK (2008a) Influence of lucerne fodder supplementation on enteric methane emission in crossbred calves. Indian J Anim Sci 78: 293-297.
- Bhatta R, Krishnamoorthy U, Mohammed F (2001) Effect of tamarind (Tamarindus indica) seed husk tannins on in vitro rumen fermentation. Anim Feed Sci Technol 90(3-4): 143-152.
- Malik PK (2006) Effect of dietary leguminous fodder on methane and nitrous oxide emission from ruminants. PhD thesis submitted to National Dairy Research Institute, Karnal, India.
- Malik PK, Singhal KK, Ahlawat A, Deshpande SB (2010) Effect of berseem fodder supplementation to wheat straw-based diet on in vitro total gas and methane production and fermentation pattern. Indian J Anim Sci 80: 551-555.
- Anantasook N, Wanapat M (2012) Influence of rain tree pod meal supplementation on rice straw based diets using in vitro gas fermentation technique. Asian-Australasian J Anim Sci 25: 325-334.
- Makkar HPS, Blümmel M, Becker K (1995) In vitro effects of and interactions between tannins and saponins and fate of tannins in the rumen. J Sci Food Agric 69(4): 481-493.
- Yogianto Y, Sudarman A, Wina E, Jayanegara A (2014) Supplementation effects of tannin and saponin extracts to diets with different forage to concentrate ratio on in vitro rumen fermentation and methanogenesis. J Indones Trop Anim Agric 39: 144-151.
- Yuliana P, Laconi EB, Wina E, Jayanegara A (2014) Extraction of tannins and saponins from plant sources and their effects on in vitro methanogenesis and rumen fermentation. J Indonesian Trop Anim Agric 39: 91-97.
- Bhatta R, Saravanan M, Baruah L, Malik PK (2017) Nutrient composition, rate of fermentation and in vitro rumen methane output from tropical feedstuffs. J Agric Sci 155(1): 171-183.
- Jayanegara A, Leiber F, Kreuzer M (2012) Meta-analysis of the relationship between dietary tannin level and methane formation in ruminants from in vivo and in vitro J Anim Physiol Anim Nutr (Berl) 96(3): 365-375.
- Malik PK, Uyeno Y, Kolte AP, Kumar R, Trivedi S, et al. (2019) Screening of phyto-sources from foothill of Himalayan mountain for livestock methane reduction. SN Applied Sciences 1: 1-9.
- Gerlach K, Pries M, Tholen E, Schmithausen AJ, Büscher W, et al. (2018) Effect of condensed tannins in rations of lactating dairy cows on production variables and nitrogen use efficiency. Animal 12(9): 1847-1855.
- Malik PK, Bhatta R, Soren NM, Sejian VA, Prasad KS, et al. (2015) Feed-based approaches in enteric methane amelioration. In: Livestock production and climate change. CABI, Wallingford, United Kingdom, pp. 336-359.
- Goel G, Makkar HP (2012) Methane mitigation from ruminants using tannins and saponins. Trop Anim Health Prod 44(4): 729-739.
- Malik PK, Kolte AP, Baruah L, Saravanan M, Bakshi B, et al. (2017b) Enteric methane mitigation in sheep through leaves of selected tanniniferous tropical tree species. Livestock Sci 200: 29-34.
- Harvey MI (2006) Unravelling the conundrum of tannins in animal nutrition and health. Journal of the Science of Food and Agriculture 86(3): 2010-2037.
- Waghorn GC, Ulyatt MJ, John A, Fisher MT (1987) The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus L. Br J Nutr 57(1): 115-126.
- Animuta G, Puchalaa R, Goetschet, AL, Patraa AK, Sahlua T, et al. (2008) Methane emission by goats consuming different sources of condensed tannins. Anim Feed Sci Technol 144: 228-241.
- Francis G, Kerem Z, Makkar HPS, Becker K (2002) The biological action of saponins in animal systems: A review. Br J Nutr 88(6): 587-605.
- Belanche A, Fuente G, Newbold CJ (2014) Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol Ecol 90(3): 663-677.
- Wallace RJ (2004) Antimicrobial properties of plant secondary metabolites. Proc Nutr Soc 63(4): 621-629.
- Malik PK, Singhal KK (2008b) Saponin content of lucerne fodder and its effect on rumen fermentation ad microbial population in crossbred bulls. Indian J Anim Sci 78: 298-301.
© 2019 PK Malik. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






