- Submissions

Full Text
Aspects in Mining & Mineral Science
Hermetic Hybrid Innovation Electrocoagulation Process for the Recovery Simultaneously Gold/Silver and HCN from Pregnant Cyanide Solution
Parga Torres JR1*, Carrollo MR1, Valdés ES1, Valdés Flores JS1, De la Peña RR1, Vázquez VV2, Etafo NO1* and Rodriguez FC3
1Department of Metallurgy and Materials Science, México
2Department of Chemical Engineering and Metallurgy, Sonora University, México
3Faculty of Engineering, Autonomous University of Coahuila, México
*Corresponding author:José Refugio Parga Torres and Nelson Oshogwue Etafo, Department of Metallurgy and Materials Science, TNM-ITS, Blvd Venustiano Carranza #2400, Col Tecnológico, Saltillo, Coahuila, C P 25280, México
Submission: October 28, 2025: Published: November 12, 2025

ISSN 2578-0255Volume14 Issue 3
Abstract
Gold and silver have diversified demand, ranging from jewelry and luxury goods to technology. Today in Mexico, the preferred method for silver and gold recovery has long been the zinc dust Merrill-Crowe process. However, the metallurgical plant’s operations must optimize every step of the precious metals recovery to remain profitable in all market conditions. The operation analysis in these plants shows that after the precious metals are precipitated with zinc dust in the filter press, the resulting cake that contain the precious metals often requires extra leaching treatment to control the accumulation of mercury, copper, lead, cadmium, and iron impurities in the final Dore product. Also, the use of cyanide in gold mining operation requires the careful handling and storage of cyanide rich tailing. Due to these operational metallurgical problems, which yields low-quality Dore (90%), along with the lengthy seven-day operation time required to obtain the precious precipitation metals cake in the filter press, high reagent costs, and prolonged treatment in the induction furnace. Recently, the Hermetic Hybrid Electrocoagulation (EC) process, is a promising alternative to achieve high-quality Dore and also attenuation between the tailings pond and the receiving environments. The EC technology requires no chemical additions for the recovery of precious metals.
Furthermore, in this study, by using air injection in the bottom of the EC reactor to remove the HCN gas and recovery simultaneously gold/silver in a Hermetic EC process and also in a separate steel reactor tank can be used to regenerate NaCN by bubbling HCN gas into liquid sodium hydroxide allowing it to be recycled back to the cyanidation leach process as a free cyanide. The optimized operational conditions voltage, current, pH, and temperature of 15V, 10A, 8, and 25 °C, respectively, the EC process yields a 98% Dore product quality within one day, compared with 7 days for the popular method, Merrill-Crowe process and recovery of cyanide of 80%. Finally, the results suggest that magnetite particles and amorphous iron oxyhydroxides present in EC remove gold and silver with an efficiency of more than 98% at an operating time of no more than 20 minutes, through magnetite species and amorphous iron oxyhydroxides generated inside the Hermetic air injection EC reactor.
Keywords:Hybrid hermetic electrocoagulation; Merrill-crowe zinc cementation process; Cyanide recovery
Introduction
In Mexico, Cyanidation is the primary technique for gold and silver ore extraction [1]. Elsner first explored gold cyanidation chemistry in 1846. In alkaline solutions containing dissolved oxygen and cyanide ions, both gold and silver undergo electrochemical reactions that lead to their dissolution as stable cyanide complexes [2] is represented by equation (1).
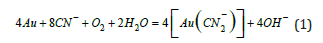
Elsner’s equation 1, while stoichiometrically valid, fails to represent the cathodic reactions in gold dissolution. Details of these electrochemical reactions are shown in equations (2) to (4):
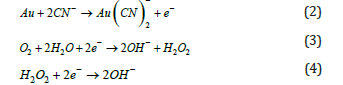
In the oxidation rate of gold, gold anodic dissolution, cyanide acts as the complexing agent and oxygen as the oxidant within the Nernst layer. Consequently, the cyanide concentration governs the anodic dissolution rate, while the dissolved oxygen concentration controls the cathodic reduction rate [3]. Figure 1 shows the schematic mechanisms of this behavior in alkaline medium. This figure shows the mechanism by which a high cyanide concentration is required to drive reaction 1 and overcome the problems with competing reactions among all the elements present. Cu, Zn, Fe, Pb, and As are the common elements. Oxygen facilitates the leaching of gold and argentite from argento pyrite by oxidizing solubilized sulfur to ferric sulfate, which then dissolves sulfides [4]. Certain elements exhibit varying oxidation states and form stable cyano complexes, as detailed by the stability constants in Table 1.
Figure 1:Photomicrography of argentopyrite particle that shows the schematic mechanism of silver and gold leaching with cyanide and activator oxygen in an alkaline medium.
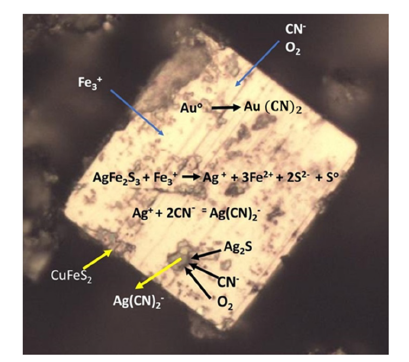
Table 1:Equilibrium constants [5].
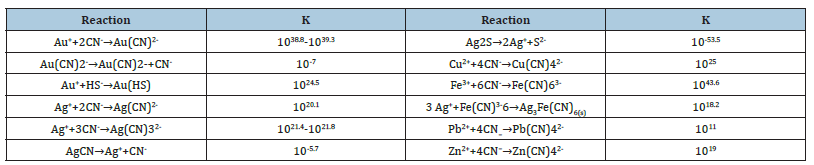
Table 1 reveals that Au, Ag, Fe, Cu, and Zn cyano complexes exhibit high stability constants. These metals increase cyanide and oxygen consumption, complicate solution purification [5], and hinder pure Dore production. Consequently, maintaining low alkali concentrations during cyanidation less than pH 11 is crucial to prevent excessive alkaline plumbite formation, which reacts with cyanide to produce insoluble basic lead cyanide [6] (see equations 5 and 6).
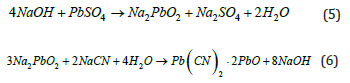
At pH levels below 10.5, reduced basic lead cyanide forms, and lead cyanide (Pb(CN)2⋅2PbO) hydrolyzes, releasing Hydrogen Cyanide (HCN). Higher concentrations of Dore in the pregnant solution generally yield higher-quality precipitates, although the presence of mercury, copper, lead, zinc, iron, and tremolite can affect this outcome [7].
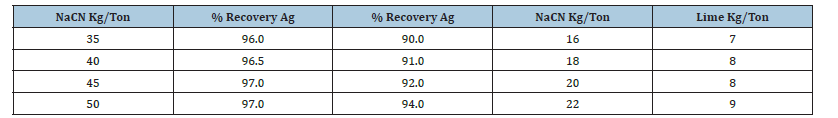
In Mexico by increasing dissolved oxygen via pressurization, this study demonstrates oxygen’s dual role: driving the reaction equilibrium and enabling mild oxidative sulfur dissolution. The resulting ferric sulfate leads to ferric ion leaching, effectively extracting gold, electrum, and argentite from refractory argentopyrite [8-10]. With this concept in mind and the potential benefits of using fine particles (40 microns). Figure 2 with high surface area, temperature no more than 80 °C, and simultaneous pressure oxidation/cyanidation leaching in the same reactor [2,8,10], we can reduce the leaching time of dissolution in 90 minutes and improve the recoveries of the precious metals in 97%. Figure 2b shows the real iron pressure reactor for gold and silver leaching by using solar/wind energy. These results show that using a particle diameter of less than 40 microns and a pressure oxidative cyanidation time of no more than 90 minutes, the dissolution of gold was 97% and of silver 94%. In Table 2, it shows the results and consumption of cyanide in the pressure leaching reactor.
Figure 2:A comparison of silver dissolution leaching using a high concentration of cyanide with simultaneous pressure of oxygen and temperature/pressure.
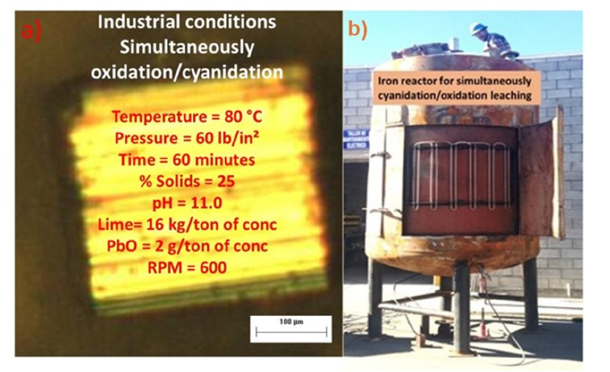
Table 2:Results of variation of cyanide concentration.
The next step is the use of Electrocoagulation process that has been known as an electrochemical phenomenon for almost a century and has been practically explored for treating waste waters. In the early treatment systems, the electric field was applied to the medium process for a short time (less than 20 minutes), and the treated dispersion transferred to a separation stage where settling occurred. The efficiency of the batch reaction has been improved by gentle agitation by either stirring or bubbling gas during the electrochemical treatment. In the latter case, a combination of electrocoagulation and flotation has been found to be an effective treatment of urban wastewater [11,12] and has also been used in conjunction with filtration to remove silica, fine activated carbon with gold [12,13] and suspended solids that tend to foul reverse osmosis membranes, thereby extending the life of the membranes.
The novelty of this study is to present a comparison of Hermetic Electrocoagulation Technology (EC), as an alternative and emerging method for the recovery of precious metals and cyanide, better than Merrill-Crowe, which has been established and well-known for more than a century for the recovery of precious metals. The final product is called Dore i.e. an alloy of gold and silver. This Hermetic EC process takes 70% less time, 50% less operational cost, and the obtained product is the recovery Dore that can be used for semiconductor application better than the Merrill-Crowe process. Finally, it is also to be noted that the hermetic reactor can be used to recover NaCN, which is the primary reagent used for the leaching of gold/silver from therefore baring of the precious metals of the Dore in the initial stage, thereby making EC a cost-effective process.
Elimination of Cyano Complex (Cyanides)
Metallurgical cyanidation uses excess cyanide beyond the Elsner equation’s stoichiometry (Table 1) to account for the dissolution of other minerals. The complexation of free cyanide readily forms with various metals, particularly transition metals, displaying a range of stabilities and solubilities. Common copper, zinc, and iron minerals, along with lead ores like galena and anglesite, dissolve in typical gold cyanidation leach solutions, forming plumbite at low alkaline concentrations [9,14,15]. The co-precipitation of metals such as copper, iron, zinc, and lead during the Merrill-Crowe process leads to the formation of low-quality precipitates. This not only increases the consumption of zinc dust but also increases the demand for smelting flux and accelerates wear on crucibles.
While hydroxide precipitation is a traditional and inexpensive method suitable for removing heavy metals present in wastewater, it suffers a crucial demerit because of its high solubility. Sulfide precipitation offers an effective alternative for recovering zinc, copper, and lead from barren cyanide solutions, achieving high metal removal across a wide pH range, though it involves the use of toxic and odorous hydrogen sulfide [16]. A key advantage of sulfide precipitation in cyanide recovery is its ability to sequentially recover zinc, lead, and copper/silver from barren solutions. Acidification of the remaining solution then releases free cyanide (HCN gas) through volatilization at pH 7-10, enabling cost-effective cyanide recycling compared to purchasing new cyanide. This cyanide regeneration process, like the nearly century-old Mills- Crowe (destruction cyanide) process developed by the Mining Company of Pachuca in Mexico (England Patent No. 241669,3.9.24) [7], relies on acid-induced dissociation of cyanide complexes, metal precipitation, and subsequent HCN volatilization (7).
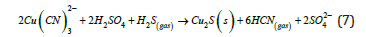
Beyond copper precipitation, sulfide treatment effectively removes nearly all silver from barren solutions in equation (8).
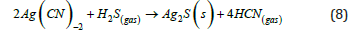
Utilizing this method, 99% copper recovery is feasible. Released HCN gas from barren solutions is stripped and then captured in a NaOH alkali solution, as depicted in the following reaction (9) :
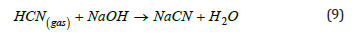
The resulting precipitate, a zinc and copper product, is marketable as is or can be combined with arsenopyrite flotation concentrate.
Industrial precipitation of cyano complex and cyanide recovery
Experimental results from a full-scale sulfide precipitation system (Serpentine) at Mining Williams, La Sorpresa and Bacis Mining Co, following the Merrill-Crowe process, demonstrate its effectiveness in recovering zinc, copper, and silver from barren solutions. The system, utilizing sodium sulfide (Na2S) as a sulfide ion source, sequentially precipitates zinc and copper/silver, while under mildly acidic conditions (pH 3-4), converting cyanide to HCN gas, as illustrated in Figure 3a-3d.
Figure 3:Two industrial applications of the precipitation of cyano complex (cyanides) and the recovery of sodium cyanide.
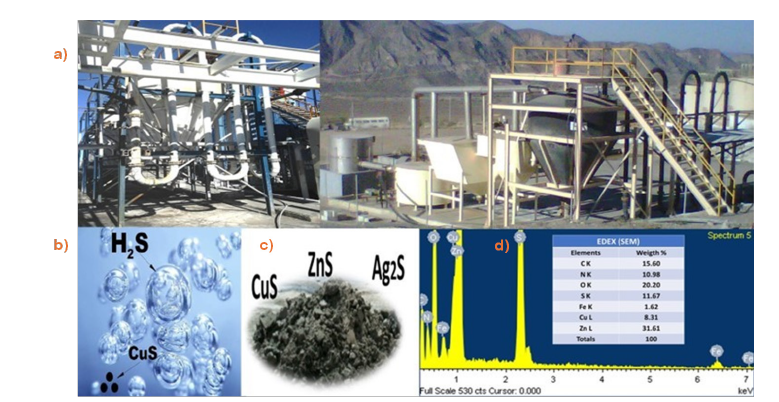
A metal cyanide precipitation process, utilizing sodium sulfide in a serpentine tube reactor with sulfuric acid pH control, effectively removes copper, zinc, iron cyanide from barren solutions. copper cyanide removal (up to 99%) occurs within a pH range of 2.5-3, with 90% recovery of cyanide associated with zinc, iron, and copper complexes. The resulting precipitate is processed by acid hot water extraction via filter-press or sun drying. Lead ions can be removed from pregnant leach solutions using natural hydroxyapatite bed filters, achieving 90% recovery. The final precipitate cake exhibits a 500% quality improvement. This technology has been successfully implemented at Minera William, La Sorpresa and Bacis Mining Company in Mexico.
Analysis of Merrill-Crowe plant
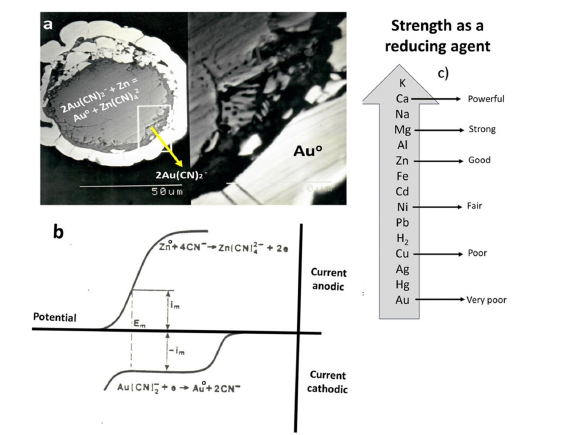
In Mexico, the Merrill-Crowe process, a zinc dust cementation electrochemical technique, is commonly used to extract Dore from alkaline cyanide solutions. This electrochemical process, involving gold reduction and zinc oxidation (Figure 4), was patented in 1894 by Sulman and Pichard and first implemented at Canada’s Del Oro mine [3,11]. In Figure 4(b), comparing Gold’s cathodic polarization behavior and silver, the cathodic overpotential for silver is much greater than that of gold, thus, the cementation of silver by zinc should be easier than the cementation of gold. These observations are supported by the results of the suspended particle’s reaction kinetics presented (1). Also, Figure 4(c) lists some common metals in a sequence called the activity series of the metals. In this arrow, any metal above hydrogen in the series can react with an acid to produce H2(g) and in a displacement reaction series, metals higher in reactivity displace metals lower in reactivity from their salt solutions. Specifically: zinc displaces copper, silver, and gold; copper displaces silver; and lead displaces gold and so forth. For example, with the activity series, we could have predicted the reaction pictured in Figure 4(a) shows the overall stoichiometry for the reaction cementation of gold with zinc is as follows:
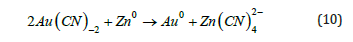
Figure 4:Illustrates the electrochemical aspects of gold cementation onto zinc dust, including (a) a simplified electrochemical model of the process. (b) Schematic current-potential curves depicting zinc anodic dissolution and gold cathodic reduction in cyanide. (c) The activity series of relevant metals (as referenced in [15]).
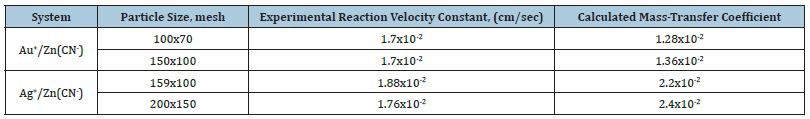
Gold cementation by zinc dust in an oxygen-free environment (<0.5 ppm O2) followed first-order kinetics, achieving over 99% gold removal (Table 3). The reaction rate exhibited an inverse first-order dependence on particle size, indicative of mass-transfer, diffusion-controlled kinetics.
Table 3:Comparison of experimental reaction velocity constants and calculated mass-transfer coefficients for suspended particles [14].
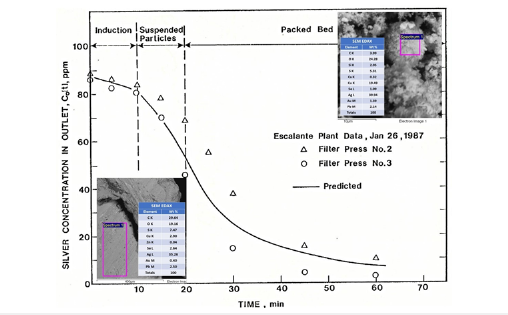
In Merril-Crowe processes, the plate and frame filter press operates in a semi-continuous mode as a fixed-bed reactor, where zinc dust and precipitated silver are collected over time. The process transitions through three distinct periods: an initial introduction period where zinc dust suspension fills the press, a brief suspendedbed reaction phase, and a prolonged packed-bed reaction phase as the filter bed grows. Plant data, supported by engineering analysis, reveals that the initial introduction and suspended-bed periods constitute only 20 minutes of the approximately seven-day cycle. A kinetic model illustrating this silver cementation behavior with zinc dust for the Escalante mine in Utah is depicted in Figure 5, [11].
Figure 5:A three-period kinetic model was developed for a semicontinuous fixed-bed reactor, reflecting the operational phases of a plate and frame filter press.
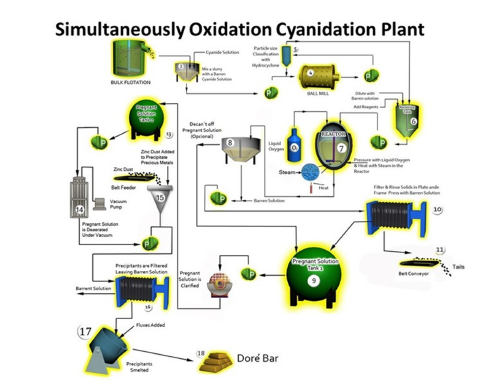
Precious metals are recovered via a flowsheet incorporating
pressure oxidation, cyanidation, and Merrill-Crowe precipitation
as shown in Figure 6. The principal steps of this metallurgical
cementation zinc dust plant are:
a. Clarification by filtration.
b. Deoxygenated in a vacuum tower.
c. Addition of zinc dust and lead salt in a mixed cone.
d. Precipitate gold and silver in a plate/frame filter press.
e. Leaching impurities of mercury, copper, lead, cadmium and
iron (basic or acid system)
f. Calcination in a furnace and smelting in the induction furnace.
g. Final Dore product.
Figure 6:Schematic general diagram of the Merril-Crowe cementation zinc dust process.
In this well-known zinc dust cementation operation to produce
final Dore typically the operation cycle is completed in 8 days.
The study focused on lead and cadmium removal during lowtemperature
acidic leaching of gold and silver calcine. This invention
focuses on selectively removing lead and cadmium, heavy metals,
from calcine leachate originating from the Merrill-Crowe process
filter press. Using an acidic or basic medium as a liquid solvent,
the leaching solution, loaded with these dissolved cadmium and
lead ions, is then treated with a filter filled with bovine bone as a
natural adsorbent to recover these toxic metals and not harm the
environment. Due to this problem, and to obtain high-quality Dore
(99%), some hydrometallurgical plants take additional steps to
achieve this goal.
A. Low-quality Dore is retreated with more fluxes and decalcified
one or more times until the desired Dore quality of 96% is
achieved.
B. If it contains cadmium or lead, the treatment method is more
critical and dangerous PbO gas, as it uses an excess of NaNO3,
intending to reduce the lead and cadmium content of the Dore
over several smelting cycles.
This study demonstrates the potential for obtaining highpurity
Dore from calcines with high lead and cadmium contents.
The use of bovine bone as an adsorbent and flux for lead and
cadmium, combined with pyrometallurgical treatments, represents
an innovation in metal extraction technology. This involves four
stages: leaching, smelting, oxygenation, and refining, resulting in
the production of high-purity Dore.
a. Leaching: The calcine, rich in gold, silver, and base metals,
undergoes a leaching process in an acidic or basic medium.
During this stage, bovine bone is used to adsorb a significant
portion of the base metals, especially cadmium and lead,
thanks to its cationic adsorption capacity.
b. Smelting: The solid resulting from the leaching is melted in
a high-temperature induction furnace. A mixture of fluxes
(NaNO₃, Na₂CO₃, borax, SiO₂, CaF₂) and additional bovine
bone are added to promote slag formation and the removal
of impurities. Figure 7(a-d) shows the total balance in each
section and the results of the Dore and slag.
Figure 7:Total balance in each section in the induction furnace and dore results.
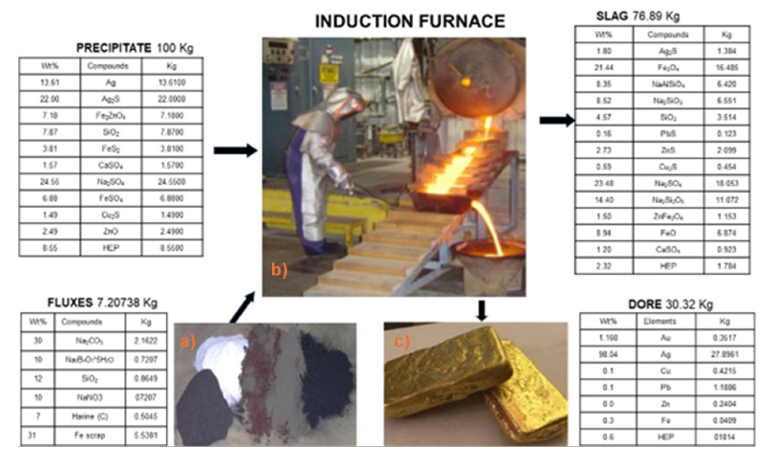
c. Oxygenation and refining: The liquid Dore undergoes an
oxidation process by oxygen/air injection at a controlled
temperature of 1000 °C. Hydroxyapatite and oxygen/air
promote the removal of metals (cadmium, lead) through slag
formation.
d. High purity dore: The final Doré obtained has a purity greater
than 99%, which increases its commercial value.
To achieve this, the precipitate will first be treated in a basic medium using hydroxyapatite or bovine bone to adsorb the lead and cadmium onto the porous bone. Hydroxyapatite will also be used as an adsorbent for lead and cadmium during smelting. This will also adsorb the remaining metallic oxides of copper and iron onto the bone, forming slag containing the highest amounts of zinc, lead, cadmium, iron, and copper impurities. Based on this innovation, it was possible to obtain a higher-purity Dore greater than 99%.
Electrocoagulation Characteristics of the Process
Electrocoagulation (EC) effectively removes ionic species, especially heavy metals, from solutions [12,13,17,18]. This Technique Uses Sacrificial Anodes (Fe or Al) to produce polyvalent cations, combined with hydrogen and oxygen gas during electrolysis processes, as illustrated in Figure 8. Electrophoresis drives negatively charged particles to the anode, while the ions that are positively charged will travel to the cathode. The released metal ions neutralize particle charges, promoting coagulation. Simultaneously, electrolysis-generated gas bubbles facilitate pollutant flotation. At the cathode, metal ions react with OH- to form insoluble hydroxides, which absorb pollutants and contribute to coagulation by neutralizing negatively charged colloids at neutral or alkaline pH, enabling agglomeration via van der Waals forces [12,17]. EC is a multi-stage dynamic process involving oxidation, reduction, decomposition, deposition, coagulation, absorption, adsorption, precipitation, froth flotation, and filtration [11,12].
Figure 8:Schematic diagram for removing gold and silver by the EC process.
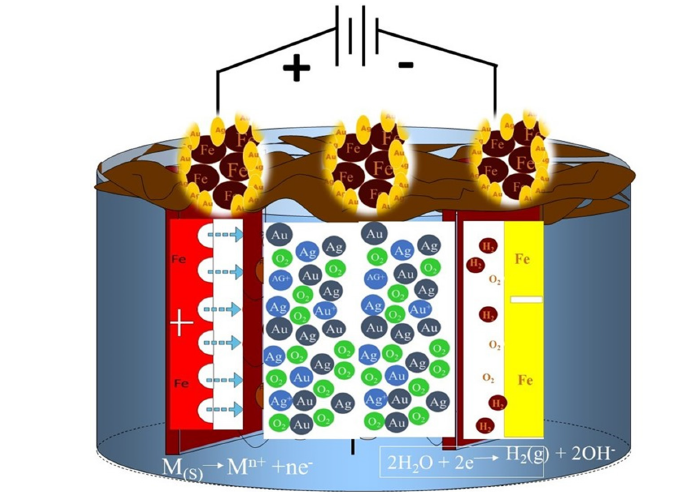
The electrocoagulation process involves a sequence of stages:
a. Electrode dissolution: Applied current releases iron ions
from the electrodes.
b. Hydroxide formation: Iron ions hydrolyze, generating metal
hydroxides and polyhydroxides.
c. Water electrolysis: Parallel reactions produce oxygen and
hydrogen gas bubbles.
d. Sludge formation: Chemical reactions lead to precipitation,
including the formation of sludge with gold, silver, and other
cations/hydroxide ions.
e. Destabilization and aggregation: Metal ions are stabilized,
particulate suspensions destabilize, emulsions break, and
aggregates (flocs) form.
f. Froth flotation: The sludge/agglomerate is removed via
natural froth flotation.
g. Filtration: Residual sludge is separated from the metal-free
aqueous solution.
However, the cycle times of all stages in the Hermetic EC process take less than 30 minutes.
Pilot plant parameters of the electrocoagulation process
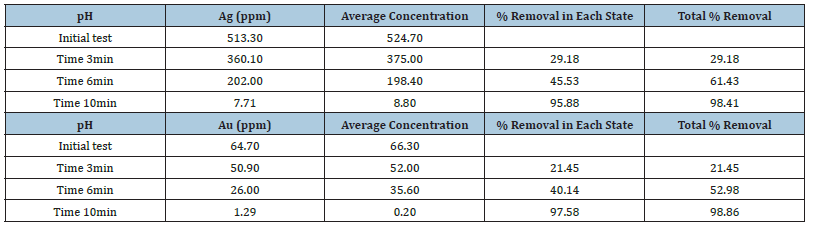
Electrochemical (EC) experiments optimized gold/silver and sodium cyanide removal from a Mexican mining (William) pregnant solution [19]. Variables tested included pH (<9), residence time, voltage, and amperage. Table 4 details the specific conditions used in each EC test with iron electrodes and in Table 5, the results for the tests performed for pH 8 and the addition of 4 grams of sodium chloride, are shown. For 10-minute residence time achieved 98% gold and silver removal. This high removal rate correlates with the production of magnetic Fe3O4 at a pH of 8-10. The magnetic properties of Fe3O4 enhance metal adsorption by physically attracting gold and silver without chemical alteration. Additionally, reduced zeta potential on iron hydroxides diminishes particle repulsion, promoting flocculation [20]. These flocs, aided by oxygen and hydrogen microbubbles generated at the electrodes, facilitate metal removal. For these experiments, the electrocoagulation pilot cell was constructed with a non-conductive transparent outer casing and iron carbon electrodes. The electrodes were spaced 1 centimeter and alternated (anode/cathode) to produce a flow of pregnant cyanide solution. Optimal cell operating characteristics were established at 25 amperes and 30 to 40 volts.
Table 4:Chemical assays and optimal parameters of EC process.
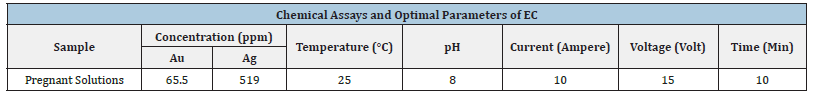
Table 5:EC Results at pH 8 and 3 different time of treatments.
A small volume of saturated NaCl brine was added to produce adequate solution conductivity. The direction of current flow was reversed automatically and performed without operator assistance approximately every 3 minutes to equalize wear on the electrodes and to remove sludge build-up from the surfaces of the electrodes. Figure 9 shows the Pilot cell EC reactor with the SEM image sludge and EDAX of gold adsorbed on magnetite species. Gold was deposited onto iron oxide/oxyhydroxide particle surfaces. The resulting gold and silver-containing sludge was separable from the depleted solution via gravity or filtration, with the depleted solution then routed to leaching cyanidation tanks for disposal [21-23].
Figure 9:Pilot plant cell reactor with the SEM image and EDAX of gold, copper, and zinc adsorbed on magnetite species.
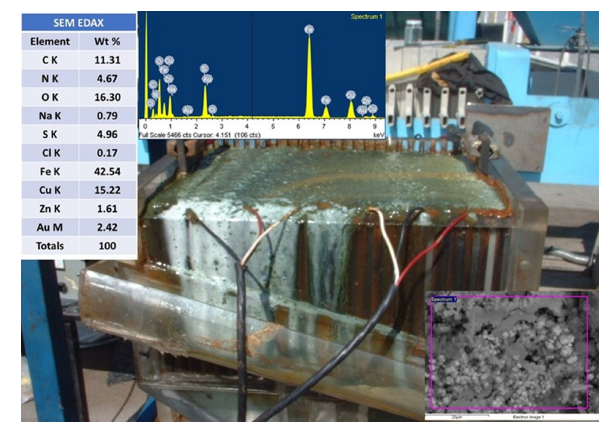
The pilot plant employed a bipolar electrode configuration, powering only the terminal iron electrodes. The intermediate electrodes were conducted via the gold and silver-containing solution. Increased power input reduced retention time, with conductivity gains offsetting sodium chloride costs. However, the system required high voltage, necessitating optimization of plate thickness and spacing. Air injection at the bottom of the reactor increase efficiency and circulation of magnetic particles and also in the reactor’s lower section removed sludge from the electrodes. From this table analysis, the EC process has shown that remove more efficiently the gold/silver ions from the pregnant solution in less operation time with cost lower than the Merrill-Crowe cementation zinc dust technology. Also, The Hematic EC process does not produce any HCN odor during the removal of gold and silver from the pregnant rich solution [24]. Additionally, no chemical reagents are required, as the coagulating agent is generated in situ by iron or aluminum electrodes.
Hermetic Removal and Recovery of Sodium Cyanide in the EC Reactor
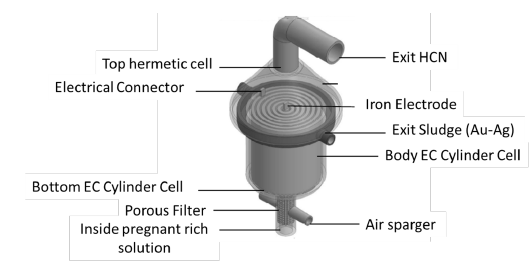
The pregnant cyanide rich solution that contains the gold and silver cyanide ions is fed tangentially at the bottom through inside surface of the porous tube, leaving an empty air core centered on the axis of the unit. The high velocity of the sparged air produces a high concentration of small bubbles and intimate interaction between these numerous fine bubbles and the pregnant rich cyanide solution. Gaseous products HCN are then transported radially to the top of the hermetic EC reactor. The major portions of the gas phase HCN move towards and are vented into an appropriate post-treatment device to recover the sodium cyanide [25]. The equipment requires a hermetic EC system for recovery gold/silver and cyanide, an operating space significantly less than that of a Merrill Crowe process, Activate Carbon, a packed tower or other air stripping devices, which result in significant savings in capital cost. The Hermetic Air Injection EC Reactor unit schematically is shown in Figure 10.
Figure 10:Hermetic hybrid electrocoagulation cell.
Procedure
Chemical analysis of cyanide in the effluent streams was accomplished with a reflux distillation method. The collected cyanide was quantified by titration with silver nitrate standard solution (Aldrich) and/or the ion selective electrode technique (Orion Model 9406). During the experiments two streams had to be delivered to the Hermetic EC reactor: the pregnant rich cyanide solution and the air. Cyanide rich solution was provided by a sump pump mounted on a 20-liter retention tank. The rich cyanide solution flow rate was adjusted using a regulated return flow to the tank. Using an air compressor, airflow was evenly distributed between the lower sections of the hermetic EC reactor. Operators were provided with personal HCN gas monitor/alarm units.
Results and Discussion
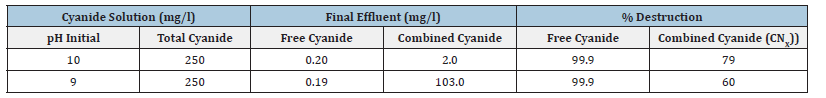
One of the primary process variables associated with cyanide destruction is the pH of the solution. Table 6 shows the results of experiment for the effect of pH on cyanide rich solution for recovery in the Hermetic EC cell. The effluent cyanide concentration (both free and combined cyanide) is shown in Table 7. The combined cyanide CNx is destroyed most effectively at high pH values more than 9 [26].
Table 6:Comparison of the electrocoagulation technology and the Merrill-Crowe Process [16,17,19,21-26].
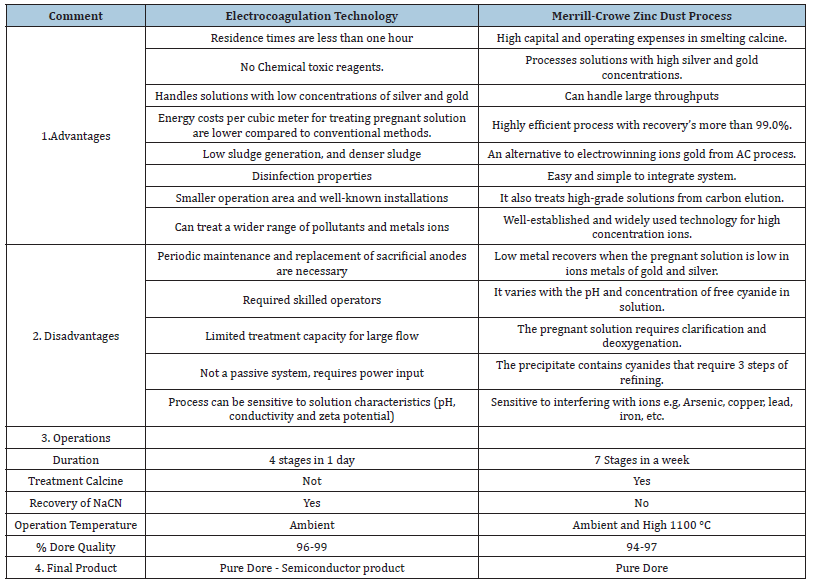
Table 7:Show the effect of pH on cyanide destruction in the pregnant cyanide. Feed solution=250mg/l total cyanide. Solution flow rate=200l/min. Air flow rate=1l/min. CNX=CNO-, HCNO, Fe(CN)6-4, etc.
Conclusion
Electrochemical Coagulation (EC) generates magnetic iron oxides (magnetite, lepidocrocite, and amorphous iron oxyhydroxides) effective for copper, cadmium and lead removal. SEM analysis confirmed the magnetic nature of these species, demonstrating copper/cadmium and lead adsorption via Van der Waal force and magnetic attraction. The EC process elevates pH, producing ferrous ions and Fe(OH)n(s) as a gelatinous suspension. This suspension facilitates gold and silver cyanide ions recovery through complexation/adsorption with the magnetic particles or electrostatic attraction, followed by coagulation and flotation. Sludge analysis revealed the composition of goethite (α-FeOOH) and maghemite γ-Fe2O3. This magnetic product has a significant semiconductor application, which has a prospect in the electronics industry.
Finally, the recovery of the precious metals in 98% and also regeneration of the sodium cyanide in 80% are economics of the Hermetic EC processes are quite attractive because of a reduced processing cost while maintaining high processing capacity and high efficiency.
Author’s Contributions
Jose Refugio Parga Torres: Conceptualization, Writing-original draft, Resources, Methodology, Investigation. Manuel Rodarte Carrillo and Esteban Sánchez Valdés: Reviewing and editing, Validation, Jesús Ventura Valdés Flores: Conceptualization, Ramon Bracamontes and Victor Vazquez Vazquez: Investigation, Nelson Oshogwue Etafo: Reviewing and editing, Validation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors gratefully acknowledge the support of CONACHYT, Bacis Mining Co and Technologic Nacional de México (Campus Saltillo and UNISON) for this Project.
References
- Reisch MS (2017) Cyanide glitters for some. C&EN Global Enterprise 95: 18-19.
- Parga JR, Valenzuela JL, Francisco CT (2007) Pressure cyanide leaching for precious metals recovery. JOM 59: 43-47.
- Miller JD, Wan RY, Parga JR (1990) Characterization and electrochemical analysis of gold cementation from alkaline cyanide solution by suspended zinc particles. Hydrometallurgy 24(3): 373-392.
- PargaTorres JR, Vázquez VV, Zamarripa GG, Etafo NO, Reyes MR, et al. (2025) Hermetic oxidization/cyanidation leaching and elimination of contaminants from the calcine for the recovery of gold and silver by electrocoagulation technology. AMMS 13(1): 1544-1553.
- Sceresini B (2005) Gold-copper ores. Developments in Mineral Processing 15: 789-824.
- Zalesov MV, Grigoreva VA, Trubilov VS, Aya B (2021) Designing of engineering solutions to enhance efficiency of high-copper gold-bearing ore processing. Mining Industry Journal (Gornay Promishlennost) pp. 51-56.
- Parga JR, Valenzuela JL, Moreno H, Pérez JE (2011) Copper and cyanide recovery in cyanidation effluents. Advances in Chemical Engineering and Science 1(4): 191-197.
- Soto-Uribe JC, Valenzuela-Garcia JL, Salazar-Campoy MM, Parga-Torres JR, Tiburcio-Munive G, et al. (2023) Gold extraction from a refractory sulfide concentrates by simultaneous pressure leaching/oxidation. Minerals 13(1): 116.
- Shi A (2024) Review of gold cyanide leaching and the main factors affecting gold dissolution rate. Naturalis Scientias 1(1): 56-60.
- PargaJ R, Valenzuela JL, Díaz AJ (2012) New technology for recovery of gold and silver by pressure cyanidation leaching and electrocoagulation. Noble Metals InTech.
- Jose Refugio PT, Esteban SV, Oshogwue EN (2024) Pollutants removal and electrocoagulation processes for high quality precipitate from cyanide-contaminated solutions. Aspect of Mining and Mineral Science 12(5): 1508-1516.
- Parga J, Cocke D, Valenzuela J, Gomes J, Kesmez M, et al. (2005) Arsenic removal via electrocoagulation from heavy metal contaminated groundwater in La Comarca Lagunera Mé J Hazard Mater 124(3): 247-254.
- Mollah MYA, Schennach R, Parga JR, Cocke DL (2001) Electrocoagulation (EC)-science and applications. J Hazard Mater 84(1): 29-41.
- Larrabure G, RodrÍguez-Reyes JCF (2021) A review on the negative impact of different elements during cyanidation of gold and silver from refractory ores and strategies to optimize the leaching process. Miner Eng 173: 107194.
- Maganga SP, Wikedzi A, Budeba MD, Manyele SV (2023) Overview of the challenges and opportunities in processing complex gold-copper ores. Min Metall Explor 40: 2463-2475.
- Zhang X, Zeng L, Wang Y, Tian J, Wang J, et al. (2023) Selective separation of metals from wastewater using sulfide precipitation: A critical review in agents, operational factors and particle aggregation. J Environ Manage 344: 118462.
- Parga Torres JR, Etafo NO, Zamarripa GG (2024) Efficient gold-cyanide recovery from activated carbon by electrocoagulation technology. Heliyon 10(21): e39570.
- Torres JRP, Etafo NO, Reyes MR, Arreguin AC (2025) Electrocoagulation flocs of torreón wastewater treatment for potential semiconductor applications. MRS Adv 10(11): 1358-1365
- Elawwad A, Hamdy A (2021) Removal of cyanide from wastewater using electrocoagulation.
- Khor CM, Liao ME, Iddya A, Ma S, Yang F, et al. (2024) Physical and electrochemical characterization of aluminum electrodes during electrocoagulation. ACS ES & T Water 4(1): 44-56.
- Moreno CHA, Cocke DL, Gomes JAG, Morkovsky P, Parga JR, et al. (2009) Electrochemical reactions for electrocoagulation using iron electrodes. Ind Eng Chem Res 48(4): 2275-2282.
- Surimbayev B, Bolotova L, Akcil A, Yessengarayev Y, Kanaly Y, et al. (2025) Technology development of gold heap leaching in kazakhstan: An overview. Mineral Processing and Extractive Metallurgy Review pp. 21-28.
- Singh S, Kapse VM, Sunil MP (2023) Gold recovery challenges from electronic waste: Opportunities and hurdles. 2023 International Conference on Power Energy, Environment & Intelligent Control (PEEIC), IEEE pp. 1344-1348.
- Kim J, Kim R, Han KN (2024) Advances in hydrometallurgical gold recovery through cementation, adsorption, ion exchange and solvent extraction. Minerals 14(6): 607.
- Etafo NO, Adekanmi DG, Awobifa OS, Torres JRP, Herrera LAI, et al. (2025) Clean and green: The multifaceted solution of the electrocoagulation technology in emerging contaminants in wastewater. Discover Civil Engineering 2: 103.
- Nkoh JN, Oderinde O, Etafo NO, Kifle GA, Okeke ES, et al. (2023) Recent perspective of antibiotics remediation: A review of the principles, mechanisms, and chemistry controlling remediation from aqueous media. Science of The Total Environment 881: 163469.
© 2025 Parga Torres JR. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






