- Submissions

Full Text
Aspects in Mining & Mineral Science
Acidipholic Bioleaching of Silver from Waste Jewellery
Diya Siji*, Lewis Robinson and Sebastien Farnaud
Centre of Health and Life Sciences, Coventry University, United Kingdom
*Corresponding author:Diya Siji, Centre of Health and Life Sciences, Coventry University, Priory Street, Coventry, Warwickshire, United Kingdom
Submission: September 17, 2024: Published: September 30, 2025

ISSN 2578-0255Volume14 Issue 2
Abstract
Silver (Ag) is a precious metal, which has a wide range of applications among a myriad of industries. The increase in the demand for silver has resulted in the depletion of primary sources of the metal. Biohydrometallurgy, also called bioleaching, a method used to recover metals using microorganisms without relying solely on chemicals, is a very promising sustainable approach for the extraction of various metals from primary and secondary sources. This study explores the use of biohydrometallurgy for recovering silver from a secondary source using comparatively Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans. The results show that the two-step bioleaching with A. ferrooxidans was more effective and resulted in a better recovery rate for silver. These encouraging results demonstrate the potential of bioleaching as a sustainable technology to recover silver from solid form, and the justification for further study.
Keywords:Bioleaching; Biohydrometallurgy; Bacteria; Silver; Oxidation; Metal recovery
Introduction
Silver (Ag), due to its physical and chemical properties, provides a crucial component in various industries, including coinage, jewelry, silverware, electronics, medical devices, confectionery, mirrors, glass, and photographic films. Additionally, thanks to its antimicrobial properties, Ag is also increasingly considered in the pharmaceutical industry [1]. However, with the rapid acceleration of urbanisation and industrialisation, the global demand for Ag led to an increase in silver-containing waste, with associated concerns regarding its sustainable supply. The extraction of Ag from the Earth’s crust is both technically challenging and environmentally costly. Consequently, recovering Ag from secondary sources such as electronic waste (e-waste), spent industrial products, and metallurgical byproducts has become critical to supplementing primary sources and ensuring a sustainable supply [2]. Ag holds significant economic value, serving not only as a strategic industrial material but also as a globally traded investment asset [3]. According to a report from The Silver Institute, industrial demand for silver is expected to increase to record-breaking heights in 2025, due to the rise in modern technologies, from renewable energy systems to advanced robotics [4]. However, primary Ag production struggles to meet this accelerating demand. To address this imbalance, enhancing the circular economy by recovering silver from secondary resources has become necessary [5].
Such approaches have motivated advancements in metal recovery technologies, including pyrometallurgy (smelting and refining at high temperatures) and hydrometallurgy (leaching and electrochemical recovery), to prioritise efficiency but with environmental sustainability. To answer these needs, Biohydrometallurgy, which uses microorganisms, is considered as a scalable and sustainable method to efficiently recover metals from waste streams, ores and concentration processes, with or without pre-concentration processes. As this method utilises leaching acids generated by microbes or through metal oxidation, it significantly reduces operational costs [6].
Acidithiobacillus ferrooxidans oxidises ferrous ions (Fe²⁺) to ferric ions (Fe³⁺), which in turn act as oxidizing agents for sulfide mineral dissolution to chemically reduced Fe³⁺ back to Fe²⁺. As the bacteria re-oxidize the resulting Fe²⁺ to Fe³⁺, a closed-loop cycle is created where the ferric ion is regenerated without requiring external replacement. This self-renewing mechanism, which demonstrates sustainability, eliminates the need for frequent oxidizing agent replenishment, a key advantage of biohydrometallurgy over traditional methods [7]. Acidophilic bacteria such as Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans are well known for their capacity to extract valuable metals using their iron-sulfur oxidising mechanism [8], but as their use for Ag recovery has not brought a lot of attention. The present study is an initial investigation of their potential use if Ag recovery.
Materials and Methods
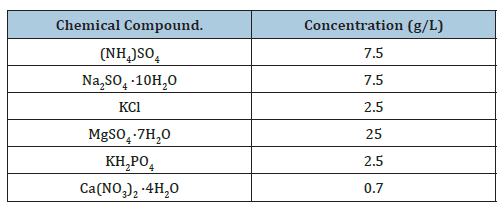
A.ferroxidans and A.thiooxidans bacteria were cultured in 100ml Acidophile Basal Salt and Trace Element (ABSTE) broth media with 30mmol Fe2+ and 1g/100ml sulfur powder at 160 RPM in the presence of a silver necklace at 1% pulp density, all conducted in sterile 250ml Erlenmeyer flaks. Bioleaching was carried out for 12 days, after which acid digestion with Nitric Acid was carried out to quantify the total amount of metals and estimate the proportion dissolved. During this period of growth, the pH was monitored by taking samples every 3 days. The concentration of silver and other metals in the Bio-leachate was determined using Inductively Coupled Plasma Mass Spectrometry (ICP-MS). ICPMS measurements were conducted using a Perkin Elmer NexION 2000 in accordance with the manufacturer’s guidelines. Cu-63 and Ag-107 were used as target isotopes for this investigation; all experiments using the ICP-MS were undertaken using He Kinetic energy discrimination (KED) to reduce mass interferences (Table 1).
Table 1:Acidophile basal salt media component concentration.
Result and Discussion
Figure 1:Comparative bioleaching recovery of silver and copper using A. ferrooxidans and A. thiooxidans in a 2- step bioleaching system. The graph shows that A. ferroxidans was highly effective for leaching silver, whereas A. thiooxidans shows very low concentrations for both copper and silver leaching, which appear to overlap due to their low concentrations.
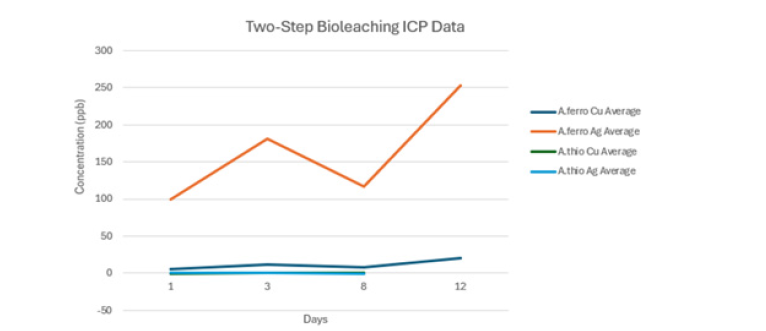
A. thiooxidans shows very low concentrations for both copper and silver leaching, which appear to overlap due to their low concentrations. In a first one-step bioleaching experiment, no solubilised metal could be detected, indicating no significant bioleaching activity. The comparative monitoring of the solubilisation of Ag using A. ferrooxidans and A. thiooxidans was conducted in a two-step bioleaching system (Figure 1), which indicates that the recovery of silver was significantly higher with A. ferrooxidans than with A. thiooxidans (Table 2). Since A. ferrooxidans can oxidise both iron and sulfur, but A. thiooxidans cannot oxidise iron, it can be suggested that the difference in Ag solubilisation with A. ferrooxidans is due to the presence of Fe3+ acting as an oxidising agent, which leads to increased solublisation of both Cu and Ag.
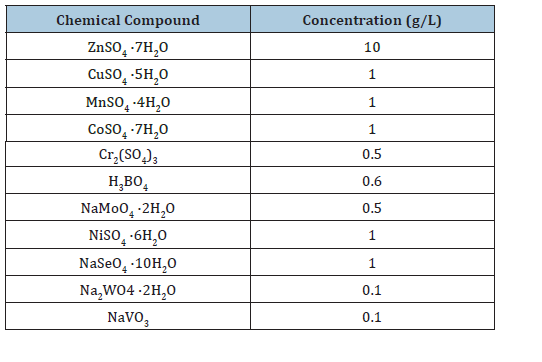
Table 2:Acidophile trace element media component concentrations.
Conclusion
The present comparative short study demonstrates that a two-step bioleaching reaction with A. ferrooxidans provides the necessary conditions for Ag solubilisation, whereas a one-step reaction, while saving time was not suitable to solubilise Ag, due to low chemical reactivity and unsuitable bacterial growth state. These encouraging initial results suggest that bioleaching technology can be used to solubilise Ag and therefore should be further investigated as a sustainable and economical method for the recovery of silver from secondary sources and diverse waste streams. Future investigations should be conducted to improve this process and develop scaled up processes adapted to industrial applications.
References
- Ghasemzadeh H, Afraz S, Moradi M, Hassanpour S (2021) Antimicrobial chitosan-agarose full polysaccharide silver nanocomposite films. International Journal of Biological Macromolecules 179: 532-541.
- Tao Q, Han C, Jing Q, Wang G (2024) Sustainable recovery of silver and copper photovoltaic metals from waste-conductive silver pastes using thiosulfate extraction and ultraviolet photolysis. Metals 14(6): 730.
- Novotný J, Polách J (2016) Real silver and its investment and business options. International Journal of Entrepreneurial Knowledge 4(1).
- Sauerwein, brandon (2025) The Silver Institute: World Silver Survey 2025. GoldSilver.
- Birloaga I, Veglio F (2018) Overview on hydrometallurgical procedures for silver recovery from various wastes. Journal of Environmental Chemical Engineering 6(2): 2932-2938.
- Chaerun SK, Winarko R, Yushandiana F (2023) Biohydrometallurgy: Paving the way for a greener future of mineral processing in Indonesia - A mini review. Current Research on Biosciences and Biotechnology 5(1).
- Tonietti L, Esposito M, Cascone M, Barosa B, Fiscale S, et al. (2024) Unveiling the bioleaching versatility of acidithiobacillus ferrooxidans. Microorganisms 12(12): 2407.
- Jones S, Santini JM (2023) Mechanisms of bioleaching: Iron and sulfur oxidation by acidophilic microorganisms. Essays in Biochemistry 67(4): 685-699.
© 2025 Diya Siji. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






