- Submissions

Full Text
Aspects in Mining & Mineral Science
A Comparison of Novel Bioleaching Approaches for the Sustainable Recovery of the Platinum Group Metals
Lewis Robinson* and Sebastien Farnaud
Centre of Health and Life Sciences, Coventry University, United Kingdom
*Corresponding author:Lewis Robinson, Centre of Health and Life Sciences, Coventry University, Priory Street, Coventry, Warwickshire, United Kingdom
Submission: September 17, 2024: Published: September 23, 2025

ISSN 2578-0255Volume14 Issue 2
Abstract
Platinum and the Platinum Group Metals (PGMs) play a vital role in industry today and in the emerging technologies of tomorrow. The PGMs are typically considered rarer than most base metals and with increased limited resource, political instability and the ever-growing climate crisis, the need for recycling these precious metals has never been greater. Traditional bioleaching technologies have been used in the mining industry since the 1940s, but due to the inert nature of the PGMs these methods of metal solubilization are considered insufficient for efficient recovery. In the quest for greener and more sustainable recycling methods, other biotechnical processes using cyanogenic bacteria and/or fungal species may provide these sustainable needed alternatives. This short review provides an insight into some emerging microbiological strategies, their advantages and limitations.
Keywords:PGM; Platinum; Bacteria; Bioleaching; Fungus; Recycling, Sustainability
Introduction
Bioleaching has been shown to be a suitable alternative to conventional chemical leaching for copper and most other base metals, which are sufficiently reactive, as shown by its applications in industry over the past 70 years [1]. However, current methods of metal processing in the mining and metal recovery industry still rely on conventional processes using potentially dangerous and environmentally damaging methodologies. In conventional hydrometallurgy, the recovery of precious metals in mining requires strong acids such as concentrated HCl and HNO3 at 4:1 ratio to extract gold, to form chloroauric acid, which can then be drained off to recover the gold while producing toxic waste [2].
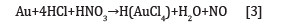
Similarly, the use of aqua regia, the mixture of nitric acid and hydrochloric acid, can be applied to recover several precious metals other than gold, including the Platinum Group Metals (PGMs) Platinum (Pt), Palladium (Pd) and Rhodium (Rh) [4]. However, the use of these strong acids has been shown to have damaging effects on the environment including acid spills, which have affected the diversity of microbial species, flora and water systems as well as some ground water supplies [5].
Pyrolysis is another conventional technique used in metal extraction, where various heat treatments are used to extract metals from a solid material. This method, which relies on the various melting points of different elements, has major drawbacks. As an energy intensive process, it uses large amounts of energy to reach the required temperatures for pyrolysis, while this process is often accompanied by the release of toxic gases such as SO2 (Malekian et al. 2019). Altogether, these examples highlight the urgent requirement for novel more sustainable applications to recover precious metals.
Bacterial Bioleaching
Commonly, the bacteria used in bioleaching are acidophilic extremophiles, including the Acidithiobacillus species such as A. thiooxidans used by Compangnone et al. [6], who were able to free the metals from ceramic, indirectly targeting the metals. Results showed that 54.5% of the Al was leached from the Al2O3 ceramic, with 24.8% being deposited in the bacterial membranes, while 0.4% of the Pt was found in the bacterial membranes. In contrast to conventional acidophile leaching, bioleaching can also be done at basic pH. This can be accomplished through a few methods, particularly with cyanide leaching as demonstrated by Karim & Ting [7]. Their research focused on the production of hydrogen biocyanide using two different bacterial species, Pseudomonas fluorescens and Bacillus megaterium. Karim & Ting [7] also used spent catalyst and targeted the Pt, Rh and Pd using a 2-step bioleaching approach. During the initial growth, better growth was observed with lower pH values (7.5, 8 and 9), therefore allowing higher production of cyanide. Karim & Ting [7] opted for a 2-step process, due to the reported toxicity of PGM compounds to the bacteria that would compromise the success of 1-step bioleaching process. They showed that the best results were obtained with P. fluorescens with 44% of Pt, 54% of Pd and 96% of Rh recovered from the samples.
Fungal Bioleaching
An alternative to bacterial bioleaching is fungal bioleaching. Pt bioleaching using fungi has been demonstrated by Malekian et al. (2019), who were able to leach Pt from spent catalysts. Malekian et al (2019) attempted bioleaching using Aspergillus niger in 1-step, 2-step and indirect bioleaching methods. The main oxidiser produced and used for leaching was oxalic acid. The process was similar to Compangnone et al. [6] with solubilization of the alumina ceramic on which the PGMs were deposited. Malekian et al (2019) tested the effects on leaching using different media, with adjusting pH with NaOH, and showed that the oxalic acid production was higher in the pH-adjusted media than in the non-adjusted media. This research also conducted a test of the effect of pulp density on the leaching rate, which was found, similarly to Karim & Ting [7], With the best results being obtained at a pulp density of be 0.5%. Under optimal biological conditions Malekian et al (2019) were able to extract 37% of the Pt from the spent catalyst. In an additional test, HCl was added to test the effect of abiotic acid, which showed only a small increase of 4% to 41% of the recovered Pt, suggesting that this initial trail of the biological process, while requiring improvement, is not far from chemical processes.
Conclusion
Bioleaching, which has been proven to be a sustainable alternative for the recovery of several precious, is also a promising alternative to chemical leaching as a green technology for PGM, although the processes require further investigation for industrial applications. As solubilization of the PGMs has been shown to be the most effective with cyanogenic bacteria, concern of toxicity remains so that fungal bioleaching might be a preferred alternative for the recovery of these precious metals. However, with increased demand in PGM, and the requirements for sustainable methods to recover metals from waste streams, these promising results with bioleaching technology deserve more attention.
References
- Natarajan KA (2018) Biotechnology of metals: Principles recovery methods and environmental concerns. 2nd (edn). Elsevier Amsterdam, Netherlands, pp. 81-106.
- Wang Y, Baker LA, Brindle ID (2016) Determination of gold and silver in geological samples by focused infrared digestion: A re-investigation of aqua regia digestion. Talanta 148: 419-426.
- Seisko EH, Junnila S, Sirvio T, Wilson T, Aromaa BP, et al. (2017) The effect of the redox potential of aqua regia and temperature of the Au, Cu and dissolution from WPCBs. Recycling 2(14): 1-9.
- Niemelä M, Pitkäaho S, Ojala S, Keiski RL, Perämäki P (2012) Microwave-assisted aqua regia digestion for determining platinum, palladium, rhodium and lead in catalyst materials. Microchemical Journal 101(1): 75-79.
- Shin D, Kim Y, Moon HS (2018) Fate and toxicity of spilled chemicals in groundwater and soil environment I: strong acids. Environmental Health and Toxicology 33(4): 180-201.
- Compagnone M, Gonzalez-Cortes JJ, Yeste MdP, Cantero D, Ramirez M (2023) Bioleaching of the a-alumina layer of spent three-way catalysts as a pretreatment for the recovery of platinum group metals. Journal of Environmental Management 345: 118825.
- Karim S, Ting YP (2022) Bioleaching of platinum, palladium and rhodium from spent automotive catalyst using bacterial cyanogenisis. Bioresource Technology Reports 18: 101069.
© 2025 Lewis Robinson. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






