- Submissions

Full Text
Aspects in Mining & Mineral Science
Effect of Electronic Concentration and Enthalpy of Mixing on the Lattice Parameter of Solid Solutions of High-Entropy Alloys Based on BCC or FCC Phases
Horban V*
Frantsevich Institute for Problems of Materials Science, National Academy of Science of Ukraine, Ukraine
*Corresponding author:Horban V, Frantsevich Institute for Problems of Materials Science, National Academy of Science of Ukraine, Ukraine
Submission: August 11, 2025: Published: August 25, 2025

ISSN 2578-0255Volume14 Issue 1
Abstract
A feature of a new class of materials-high-entropic alloys - is the presence of atoms with different properties and the absence of a dominant element and in accordance with the law of self-organisation this should lead to the appearance of new dependences characteristic only for high-entropic alloys. Identification of new dependences inherent to high-entropy alloys is one of the important in the study of high-entropy alloys. Analyses of single-phase high-entropy alloys have revealed that the lattice parameter depends significantly on the electron concentration. In the range of electron concentration up to 7,3el/at, alloys with BCC crystal lattice are formed. High-entropy alloys with FCC crystal lattice are formed at electron concentration higher than 7,8el/at. It is also revealed that for these alloys the shift of the enthalpy of mixing towards negative values is accompanied by a decrease in the lattice parameter. It is revealed that in the presence of positive enthalpy of mixing in single-phase high-entropy alloys, the determined lattice parameter will always be greater than the calculated one. And only at total negative enthalpy of mixing and the presence of a pair with negative enthalpy of mixing above -40kJ/mol in such alloys the determined lattice parameter is less than the calculated one. The peculiarities of single-phase high-entropy alloys include the dependence of the elastic modulus on the lattice parameter.
Keywords:High-entropy alloys; Electron concentration; Hardness; Elastic modulus; Lattice parameter; Enthalpy of mixing
Introduction
A new class of materials - High-Entropy Alloys (HEA) due to the presence of atoms with different electronic concentration, radius size and elastic modulus - forms the phase composition and physical and mechanical properties based on new factors. The study of a large number of single-phase VES has revealed a significant role of electron concentration in the formation of phase composition [1-5]. It is also shown that single-phase HEA are formed only in certain intervals of mixing enthalpy and atomic mismatch (lattice-distortion) [6-9]. The role of distortion in hardening [10-12], and of the lattice parameter on the elastic modulus has been noted [13,14]. All this contributes to the fact that in the state of single-phase solid substitution solution HEA exhibit increased strength values in a wide temperature range [6,15-19].
Analysis of publications shows that in most single-phase HEAs, the fitted lattice parameter is larger than that calculated by the mixture rule [15,16]. However, in some cases, it is noted that the determined lattice parameter of single-phase HEA with a BCC crystal lattice is smaller than that calculated by the mixture rule [15,16]. The aim of this work is to analyses the available data on the modulus of elasticity and lattice parameter of single-phase HEA to identify the factors affecting their parameter [20].
Materials and Methods
The compositions of solid-solution HEAs based on BCC or FCC phases in which the lattice parameter, elastic modulus and hardness were determined were taken from [14,16,18-23]. The averaged values of the electron concentration parameter (number of valence electrons per Csd atom), atomic radius (rmix.), and elastic modulus (Emix.) were calculated using the mixture rule.
Distortion () was estimated using the formula:
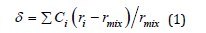
where: ci is the concentration of this kind of atoms, respectively, ri is the atomic radius of the i atoms, rmix is the average atomic radius of the alloy elements.
In experiment, as it is known, the lattice parameter of metals [23], including high-entropy alloys, is determined. For pure metals with BCC or FCC crystal lattice, the density of their filling has been established. On this basis, the calculated lattice parameter of the high-entropy alloy based on the FCC crystal lattice was calculated as a(HEA)FCC= rmiхx2.83, and on the basis of the ВCC crystal lattice - a(HEA)BCC= rmiхx2.31.
The value of the enthalpy of mixing was calculated as a linear combination of the interaction energies between pairs of atoms included in the alloy according to the formula:
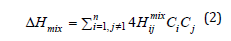
where Hijmix is the enthalpy of mixing of two atoms i and j, calculated within the framework of the Miedema model [24]. The data for the subsequent calculation are taken from [25]. Table 1 shows the characteristics of the elements included in the studied HEA that are used for calculations. Table 2 Compositions of HEAs based on solid solutions with BCC or FCC crystal lattice and their characteristics obtained in the experiment (a, H, E), the rest by calculation. The alloys are arranged in the order of increasing electron concentration.
Table 1:Characteristics of the elements included in the composition of the studied HEA.
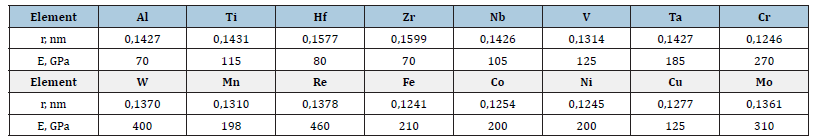
Table 2:Presents the experimental [14,16,18-23] and calculated characteristics of high-entropy alloy based on solid solutions HEA with BCC or FCC crystal lattice.
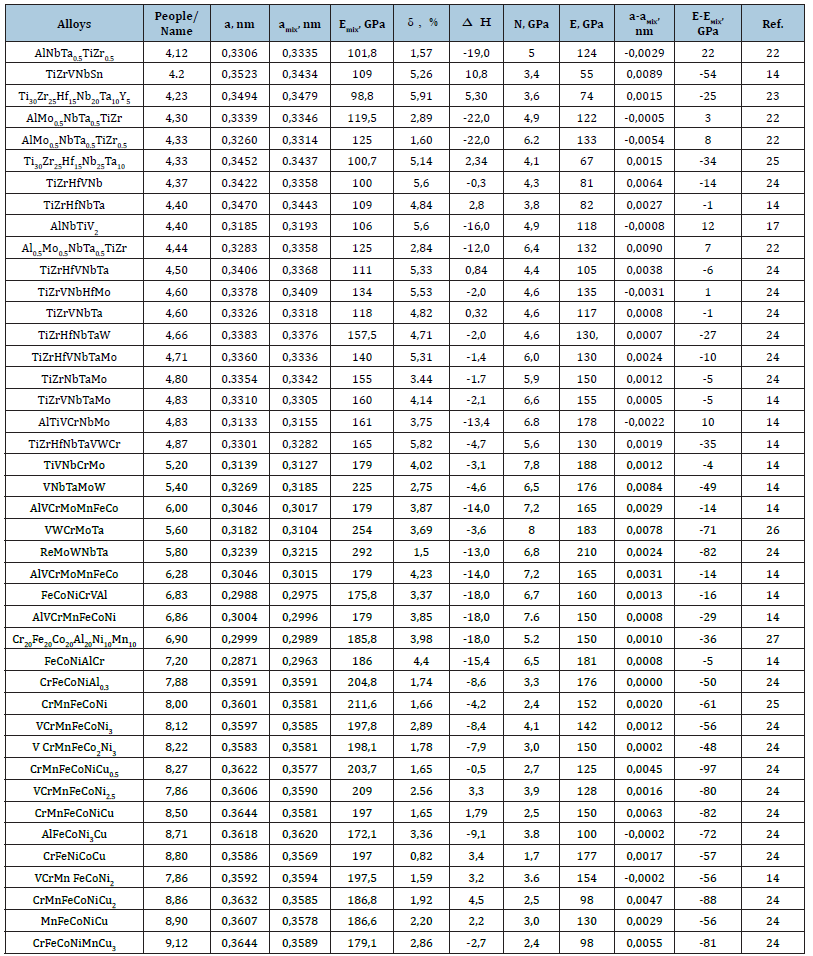
The analysis of the presented data in Table 2 shows that some solid solutions of HЕАs containing Al, Ti, Zr are characterized by mixing enthalpy values higher than - 15el/at. The composition of HЕАs includes atoms with different electronic concentrations. Therefore, it is of interest to reveal general tendencies of the influence of electron concentration on the lattice parameter of solidsolution high-entropy HЕАs in the range of electron concentration from 4.12 to 9.12el/at.
Figure 1:Character of variation of the lattice parameter of НЕА based on solid solutions with BCC or FCC phases as a function of electron concentration.
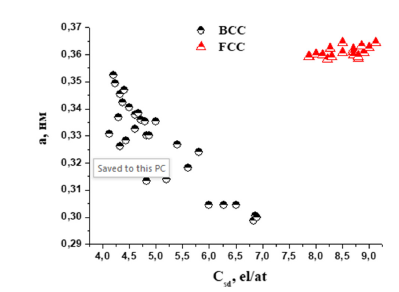
As can be seen from Figure 1, there is a relationship between the electron concentration and the lattice parameter of HEA alloys based on BCC or FCC phases. With increasing electron concentration, the character of the lattice parameter change for HEA with BCC or FCC phases is opposite. For HEA based on solid solutions with BCC phases, with increasing electron concentration there is a downward shift of the lattice parameter values. And for HEA based on solid solutions with FCC phase, an increase in the values of the lattice parameter with increasing electron concentration is observed. In addition to the influence of electron concentration on the formation of the lattice parameter of HEAs, it is necessary to take into account such factors as the presence of mixing enthalpy in HEAs, which largely determines the strength of interatomic chemical bonds.
The data presented in Figure 2 show that for solid solutions with both BCC or FCC phases, an increase in the lattice parameter is recorded when the enthalpy of mixing is shifted towards positive values. For solid solutions with BCC lattice this influence is more visible. The work [13] noted the role of the lattice parameter in shaping the physical and mechanical properties of solid solutions of HEA based on the BCC phase. Indeed, as can be seen in Figure 3, for most alloys, an increase in the elastic modulus is observed with a decrease in the lattice parameter in single-phase HEAs based on the BCC or FCC phases.
Figure 2:Effect of enthalpy of mixing in solid solutions of HEAs with BCC or FCC phases on their lattice parameter.
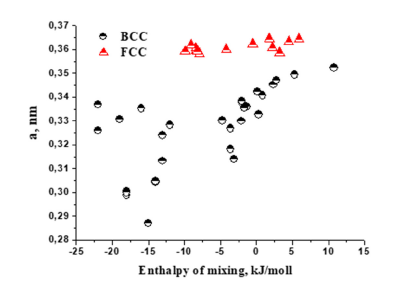
Figure 3:Effect of lattice parameter on the elastic modulus of single-phase HEAs based on both BCC and FCC phases.
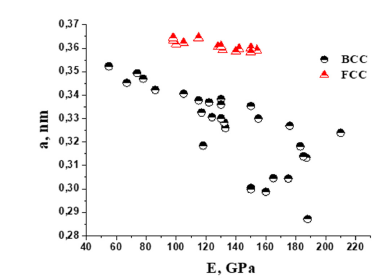
Figure 4:Effect of mixing enthalpy on the difference of lattice parameters of single-phase HEA determined in experiment and calculated by the mixture rule.
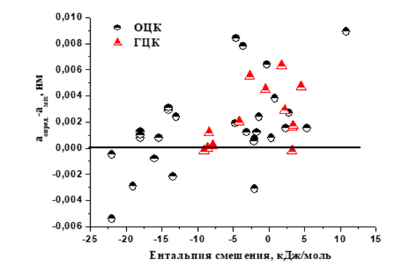
The analysis of Table 2 shows that in some cases the determined modulus of elasticity is greater than the calculated modulus of elasticity. At the same time, the calculated lattice parameter is smaller than that determined in the experiment. In high entropy alloys, factors such as distortion and enthalpy of mixing affect the lattice parameter in addition to electron concentration. An increase in the distortion level favors an increase in the lattice parameter, while a decrease in the negative enthalpy of mixing favors a decrease in the lattice parameter. In Figure 4 shows the effect of mixing enthalpy on the difference of lattice parameters of singlephase HEA determined in the experiment and calculated by the mixture rule.
The presented data show that in the presence of the total negative enthalpy of mixing in the BCC phase, the lattice parameter determined in the experiment is lower than the calculated one. For НЕА with FCC phase, only the presence of a general negative enthalpy of mixing and a pair with a negative enthalpy of mixing above -40kJ/mol leads to the fact that the determined lattice parameter is lower than the calculated one.
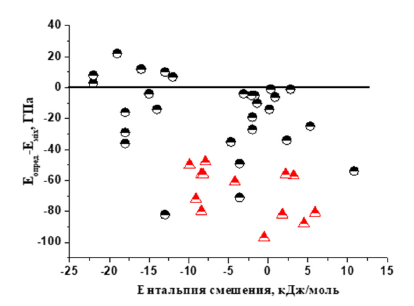
A similar effect of mixing enthalpy has a similar effect on the difference of elastic moduli of single-phase HEA determined in experiment and calculated by the mixture rule (Figure 5). As can be seen from Figure 5, the elastic modulus determined in experiment can be larger than the calculated one only in HEA with BCC phase. For HEA with FCC phase, the elastic modulus determined in experiment is always smaller than the calculated one, irrespective of the enthalpy of mixing.
Figure 5:Effect of mixing enthalpy on the difference of elastic moduli of single-phase HEA determined in the experiment and calculated by the mixture rule space.
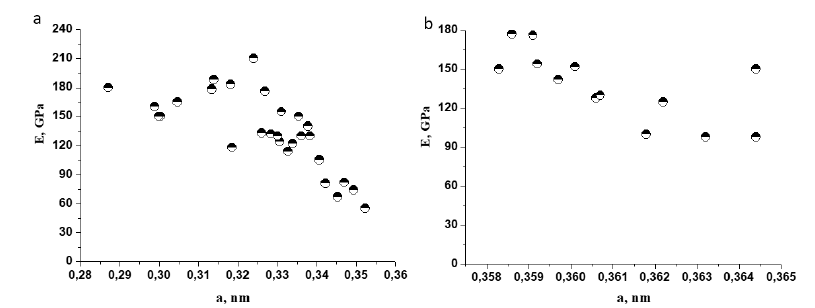
It was noted earlier [22] that the lattice parameter in singlephase HEAs affects the elastic modulus. Indeed, the results presented in Figure 6 results show that for single-phase HEA, a decrease in the elastic modulus with increasing lattice parameter is observed. For solid-solution HEA based on BCC crystal lattice (Figure 6 a), it can be seen that the elastic modulus increases proportionally with decreasing lattice parameter. It changes from 40 to 210GPa when the lattice parameter decreases by 0.07nm. For single-phase HEAs with FCC crystal lattice (Figure 6b), an increase in the elastic modulus from 98 to 180GPa is observed when the lattice parameter is reduced by only 0.007nm.
Figure 6:Effect of the lattice parameter of single-phase BCC (a) and FCC (b) HEA on the elastic modulus.
Conclusion
It is revealed that with increasing electron concentration the character of the lattice parameter changes for high-entropy alloys based on solid solutions with BCC and FCC phases is opposite. It is established that the shift of mixing enthalpy characteristics towards negative values in single-phase HECs is accompanied by a decrease in the lattice parameter. It is revealed that in the presence of a general negative enthalpy of mixing in HEA with FCC phase, the lattice parameter determined in the experiment is lower than the calculated one. At the same time, for HEA with FCC crystal lattice, the elastic modulus determined in the experiment is always lower than the calculated one, irrespective of the mixing enthalpy level. For HEA with a BCC crystal lattice, only the presence of a total negative enthalpy of mixing and a pair with a negative enthalpy of mixing above -40kJ/mol leads to the fact that the determined elastic modulus is higher than the calculated one. The dependence of the elastic modulus on the lattice parameter in single-phase HEA is shown.
References
- Zhang Y, Zhou YJ (2007) Solid solution formation criteria for high entropy alloys. Mater Sci Forum 561-565: 1337-1339.
- Senkov ON, Wilks GB, Miracl DB, Chuang CP, Liaw PK (2010) Refractory high-entropy alloys. Intermetallics 18(9): 1758-1765.
- Guo S, Ng C, Lu J, Liu CT (2011) Effect of valence electron concentration on stability of FCC or BCC phase in high entropy alloys. Journal of Applied Physics 109: 103505.
- Yang X, Zhang Y (2012) Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater Chem Phys 132(2-3): 233-238.
- Firstov SA, Gorban VF, Krapivka NA, Karpets MV, Pechkovskii EP (2016) Effect of electron density on phase composition of high entropy equiatomic alloys. Powder Metallurgy and Metal Ceramics 54: 607-613.
- Yeh JW (2006) Recent progress in high entropy alloys. Ann Chim Sci Mater 31: 633-648.
- Otto F, Yang Y, Bei H, George EP (2013) Relative effects of enthalpy and entropy on the phase stability of equiatomic high entropy alloys. Acta Mater 61(7): 2628-2638.
- Gorban VF, Firsov SO, Krapivka MO (2023) Іnfluence of electron concentration, mixing enthalpy and lattice distortion on the physical and mechanical properties of high-entropy alloys with an FCC lattice. Physico-Chemical Mechanics of Materials 2: 1-7
- Wang Z, Fang Q, Li J, Liu B, Liu Y (2018) Effect of lattice distortion on solid solution strengthening of BCC high-entropy alloys. Mater Sci Technol 34(2): 349-354.
- Poplawsky S, Li S, Samaei AT, Lee C, Song G, et.al. (2018) Lattice distortion in a strong and ductile refractory high-entropy alloy. Acta Mater 160: 158-172.
- Li L, Fang Q, Li J, Liu B, Liu Y, et al. (2020) Lattice-distortion dependent yield strength in high entropy alloys. Materials Science and Engineering A 784: 139323.
- Gorban VF, Firsov SO, Krapivka MO, Samelyuk AV, Kurylenko DV (2022) Influence of various factors on the properties of solid-soluble high-entropy alloys based on BCC and FCC phases. Materials Science 58: 135-140.
- Firstov SA, Mileiko ST, Gorban VF, Krapivka NA, Peczkowski EP (2014) Elasticity modulus of high entropy single-phase alloys with BCC crystal lattice. Composites and Nanomaterials 1(6): 3-17.
- Gorban VF, Krapivka NA, Firstov SA (2017) High-entropy alloys: Interrelations between electron concentration, phase composition, lattice parameter, and properties. Physics of Metals and Metallography 118(10): 970-981.
- Podolskiya AV, Tabachnikova ED, Voloschuka VV, Gorban VF, Krapivka NA, et al. (2018) Mechanical properties and thermally activated plasticity of the Ti30Zr25Hf15Nb20Ta10 high entropy alloy at temperatures 4.2-350 K. Materials Science and Engineering: A 710: 136-141.
- Xu H, Hu M, Zhu D, Wang T, Jiang W, et al. (2025) An investigation on high-temperature properties of a lightweight AlNbTiV2 refractory high-entropy alloy reinforced by Si. Intermetallics 180: 108705.
- Tseng KK, Huang HH, Wang WR, Yeh JW, Tsai CW (2022) Edge-dislocation-induced ultrahigh elevated-temperature strength of HfMoNbTaW refractory high-entropy alloys. Science and Technology of Advanced Materials 23(1): 642-654.
- Gorban VF, Andreev AA, Stolbovy VA, Firstov SA, Karpets MV, et al. (2023) Properties of metal, nitride, oxide, and carbide coatings produced from high-entropy alloys. Powder Metallurgy and Metal Ceramics 62(7-8): 469-480.
- Kim HS, Praveen S (2018) High-entropy alloys: Potential candidates for high-temperature applications–An overview. Adv Eng Mater 20(1): 1700645.
- Gorban VF, Krapivka NA, Firstov SA (2019) The role of mixing enthalpy in the formation of physico mechanical properties of solid solution highly entropic alloys. Electron Microscopy and Strength of Materials-Kiev: Frantsevich Institute for Problems of Materials Science NASU 25: 8-162019
- Senkov ON, Jensen JK, Pilchak AL, Miracle DB, Fraser HL (2018) Compositional variation effects on the microstructure and propertiesof a refractory high-entropy superalloy AlMo5NbTa0.5TiZr. Materials and Design 139: 498-511.
- Horban VF (2024) Designing of solid solution high entropy alloys with BCC of FCC crystal structures. J of Materials Engineering 52(1): 16-26.
- Gorban VF, Krapivka NA, Firstov SA, Kurilenko DV (2018) Role of various parameters in the formation of the physicomechanical properties of high-entropy alloys with BCC lattices. Physics of Metals and Metallography 119(5): 477-481.
- Gorban VF, Karpets MV, Makarenkoa YS, Kostenko AD, Danylenko NI (2015) Friction of high-entropy Fe25Cr20Ni20Mn15Co10Al10 alloy for 65G steel. Journal of Friction and Wear 36(4): 342-345.
- Miedema AR, de Chatel PF, de Boer FR (1980) Cohesion in alloys-fundamentals of a semi-empirical model. Physica100 BC 1: 1-28.
© 2025 Horban V. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






