- Submissions

Full Text
Aspects in Mining & Mineral Science
Studies on the Impacts of Excavation of Mineral Contact Sediments on the Behaviour of Steam Coals
René Luhmer1, Manuela Neuroth1* and Helge Stanjek2
1RWE Power AG, Research & Development, Power Plant Technology & Storage, Germany
2RWTH Aachen, Institute for Clay and Interface Mineralogy, Germany
*Corresponding author: Manuela Neuroth, RWE Power AG, Research & Development, Power Plant Technology & Storage, Mineralogy, Werkstraße, 50129 Bergheim, Germany
Submission: January 19, 2022;Published: January 26, 2022

ISSN 2578-0255Volume8 Issue4
Opinion
During lignite exploitation in the Rhenish lignite district, a low excavation of sediments (sands and clays) neighboring the lignite cannot be completely avoided. Thereby, the chemical and mineralogical compositions as well as the combustion residues are modified, if those contaminated lignite is used as steam coal, and it is possible that it shows other properties during combustion with regard to its propensity of developing undesired accumulations in boilers, such as sinter or slacks, in comparison to pure lignite. In the Rhenish lignite district, there are three opencast mining spots still in operation: Garzweiler mine, Hambach mine (Figure 1) and Inden mine. Although the mining spots are not far away from each other in spatial distance and the seams have similar ages, they vary significantly in their chemical composition because of their different environment during deposition and formation. Therefore, the adjacent sediments also vary with their chemical and mineralogical compounds within and between the mining spots. Samples from lignite and their directly adjoining sediments were taken in all three mining spots to study the differences of the behaviour during combustion between pure and contaminated lignite in laboratory experiments. Before simulating the excavation of sediments during lignite exploitation by mixing sediments with lignite in certain ratios, all pure materials were characterised by determining the ash contents (at 450 °C and 815 °C), ash conversion temperatures (hot stage microscope) as well as the chemical (X-Ray fluorescence-XRF) and mineralogical (X-Ray diffraction-XRD) compositions of the incineration residues (from 815 °C incineration). The mixtures were made of incineration residues of lignite and sediments. They were mixed in a ratio simulating an excavation of 10cm, respectively 20cm, of adjacent sediment in addition to lignite by a bucket wheel excavator. Pure and mixed incineration residues were used for running temperature and time dependent experiments. For determining ash conversation temperatures, a hot stage microscope from Hesse Instrumente e.K. was used. Experiments were run following the descriptions of DIN 51730 [1]. XRF analyses were determined using an S8 Tiger, provided with a wavelength dispersive spectrometer, by BRUKER AXS GmbH. It is equipped with a rhodium tube and has a maximum output of 4kW. Measurements were performed under vacuum, a collimating mask with 34mm in diameter as well as a rotation of the sample by a half revolution per second. XRD-analyses were run on a D4 Endeavor by Bruker AXS GmbH, equipped with copper tube. Measurements were performed with a nickel filter, fixed divergence slit and Lynx Eye detector for a measurement range from 6 to 80 °2θ. For the determination of the amorphous content of the samples, an amount of corundum of 20 wt% was added to every sample after running the heating experiments. For quantitative analyses BGMN [2] and Profex [3] were used.
Figure 1: Bucket wheel excavator in Hambach mine extracting lignite in a deep cut.

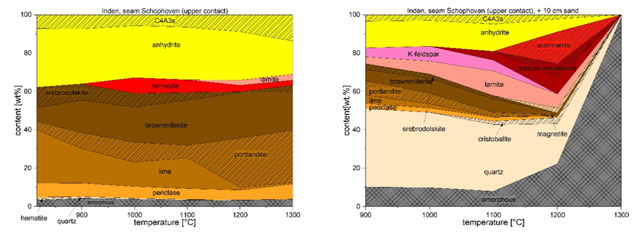
All combustion residues contain primary and secondary ash constituents. Quartz (SiO2) can be identified in every incineration residue from Rhenish lignite and belongs to the primary ash constituents because it is formed before combustion. Other primary components are rutile and anatase. Anhydrite (CaSO4) is also found in every residue from Rhenish lignite, but it is formed only during combustion and belongs to the secondary ash constituents. Other secondary compounds are for example lime (CaO), periclase (MgO), brownmillerite (Ca2(Al, Fe3+)2O5) or hematite (Fe2O3). Depending on the content of quartz, on the presence of other elements in the sample, especially alkali metals, and with rising temperature the transition from quartz to cristobalite can be observed. With increasing temperatures, the amount of anhydrite decreases. While sulphur can escape as SO3, calcium is used for the building of other minerals such as anorthite (Ca [Al2Si2O8]), larnite (Ca2SiO4), akermanite (Ca2Mg [Si2O7]), diopside (CaMg [Si2O6]) and (pseudo-) wollastonite (CaSiO3). Quartz is acting as a possible reaction partner for the formation of those minerals (Figure 2). The occurrence of those phases indicates to high temperature zones in boilers and can be used as indicators for the beginning of the formation of solid accumulations which can lead to slacks. In samples without a notable concentration of aluminium the formation of ternesite (Ca5[SO4|(SiO4)2]) takes place at temperatures from 900 to 1000 °C (Figure 2). Ternesite can be used as a tracer for the transition zone from solid siliceous accumulations to looser sulphatic deposits on boiler components. Due to the addition of mineral contact sediments during the excavation either the content of quartz (for sands) or of amorphous components (for clays) rises. With increasing the temperature for sandy mixtures, the rise of either cristobalite or silicates can be observed. By raising the temperature in clayey mixtures, the formation of mullite can be identified while the number of amorphous constituents decreases. In total, the result of these studies shows significant accordance of reconstructed decomposition reactions, solid-solid-reactions, liquid-solid-reactions up to complete melting with analytical verified mineral reactions and the tendency of the formation of unwanted accumulations in lignite fired boilers from RWE Power AG. Therefore, mineral reactions in steam coals, with different chemical and mineralogical compositions, can be associated to certain temperature spectra. This makes it possible to derive information on the formation temperature by investigating the mineral composition of boiler accumulations if the sampling location is known.
Figure 2: Mineralogical compositions of the incineration residue from the roof contact lignite of seam Shophoven, Inden mine, in dependence of the temperature (left side) as well as compositions of the mixture of the lignite incineration residue from seam Shophoven, Inden mine, with adjacent sand (10cm) in dependence of the temperature (right side). Portlandite was formed during cooling the samples from lime. It is not formed during the heating experiments.
References
- DIN 51730 (2007) Solid fuel testing - determination of ash melting behavior.
- Bergmann J, Friedel P, Kleeberg R (1998) BGMN-A new fundamental parameters-based Rietveld program for laboratory X-ray sources, its use in quantitative analysis and structure investigations. CPD Newsletter 20: 5-8.
- Doebelin N, Kleeberg R (2015) Profex: A graphical user interface for the Rietveld refinement program BGMN. Journal of Applied Crystallography 48(5): 1573-1580.
© 2022 Manuela Neuroth. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






