- Submissions

Full Text
Associative Journal of Health Sciences
Epigastric Pain as a Clue to Myopericarditis Revealing Eosinophilic Granulomatosis with Polyangiitis: A Case Report
Julien Quillet*
Department of Cardiology, Centre Hospitalo-Universitaire de Caen, France
*Corresponding author:Julien Quillet, Department of Cardiology, Centre Hospitalo-Universitaire de Caen, France
Submission: July 21, 2025;Published: July 29, 2025

ISSN:2690-9707 Volume4 Issue2
Abstract
Background: Cardiac involvement in Eosinophilic Granulomatosis with Polyangiitis (EGPA), formerly
Churg-Strauss syndrome, is a rare but severe manifestation. Early diagnosis and immunosuppression are
critical to avoid irreversible endomyocardial fibrosis.
Case Summary: A 36-year-old asthmatic woman presented with recurrent epigastric and a typical chest
pain for several weeks. Initial gastrointestinal work-up was unremarkable. Transthoracic echocardiography
showed a mild circumferential pericardial effusion with preserved left ventricular ejection fraction.
Laboratory tests revealed marked hyper eosinophilia (9G/L), high CRP (85mg/L), and rising high-sensitivity
troponin I (248→812ng/L). Within 48 hours, clinical deterioration occurred with worsening eosinophilia
(36G/L), purpuric skin lesions, episcleritis, and peripheral neuropathy. EGPA related myopericarditis
was suspected. High-dose corticosteroids (1mg/kg) were initiated urgently, leading to spectacular
clinical and biological improvement within 24 hours. Cardiac magnetic resonance imaging confirmed
acute myopericarditis with diffuse myocardial edema and pericardial enhancement.
Discussion: This case emphasizes that in asthmatic patients with unexplained hyper eosinophilia, even
atypical chest or epigastric pain should raise suspicion of eosinophilic myocarditis or EGPA. Early immunosuppressive
therapy is essential to prevent life-threatening complications.
Keywords:Eosinophilic Granulomatosis with Polyangiitis; Myopericarditis; Churg-Strauss syndrome; Hyper eosinophilia
Introduction
Eosinophilic Granulomatosis with Polyangiitis (EGPA), previously known as Churg-Strauss syndrome, is a systemic necrotizing vasculitis characterized by asthma, hyper eosinophilia, and multi-organ involvement. Cardiac manifestations occur in up to 40% of patients and are a major cause of mortality, mainly due to eosinophilic myocarditis and endomyocardial fibrosis [1]. We report a case of EGPA revealed by recurrent epigastric pain and acute myopericarditis.
Case Presentation
Initial presentation
A 36-year-old woman with a history of childhood asthma, treated intermittently with inhaled bronchodilators, presented with recurrent epigastric and atypical chest pain evolving over 10 months. Gastrointestinal work-up, including gastroduodenoscopy and abdominal ultrasound, was unremarkable. A thoracoabdominal CT scan performed for persistent symptoms revealed a moderate pericardial effusion, confirmed by outpatient transthoracic echocardiography (TTE).
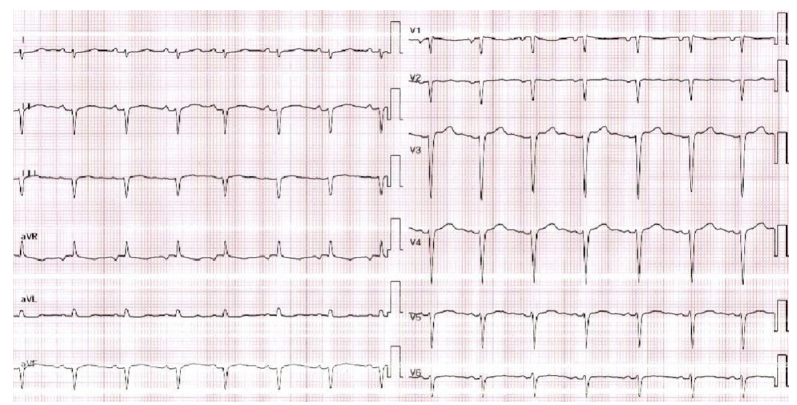
On admission, the patient was afebrile (37 °C) with sinus tachycardia (108bpm), normal blood pressure (130/82mmHg), and no heart failure signs. Physical examination showed only epigastric tenderness. ECG revealed sinus rhythm, low QRS voltage in limb leads, and anterior R-wave attenuation (Figure 1). TTE demonstrated preserved left ventricular ejection fraction (LVEF 60%), normal Nilling pressures, and a circumferential 1-mm pericardial effusion.
Fgure 1:ECG revealed sinus rhythm, low QRS voltage in limb leads, and anterior R-wave attenuation.
Laboratory findings
Initial laboratory results showed leucocytosis (19.9G/L) with marked hyper eosinophilia (9.1G/L), elevated C-reactive protein (85mg/L), and rising high-sensitivity troponin I (248ng/L to 812ng/L over 48h). Renal function was normal.
Clinical deterioration and diagnosis
Within 48 hours, the eosinophil count increased dramatically to 36G/L. The patient developed purpuric lesions on the hands, episcleritis, and painful peripheral neuropathy. Retrospective history revealed recent worsening of asthma.
Given the constellation of asthma, hyper eosinophilia, and systemic involvement, EGPA- related myopericarditis was strongly suspected.
Autoimmune and infectious work-up showed negative ANCA, ANA, and parasitological studies. Bone marrow aspirate ruled out haematological malignancy.
Management and outcome
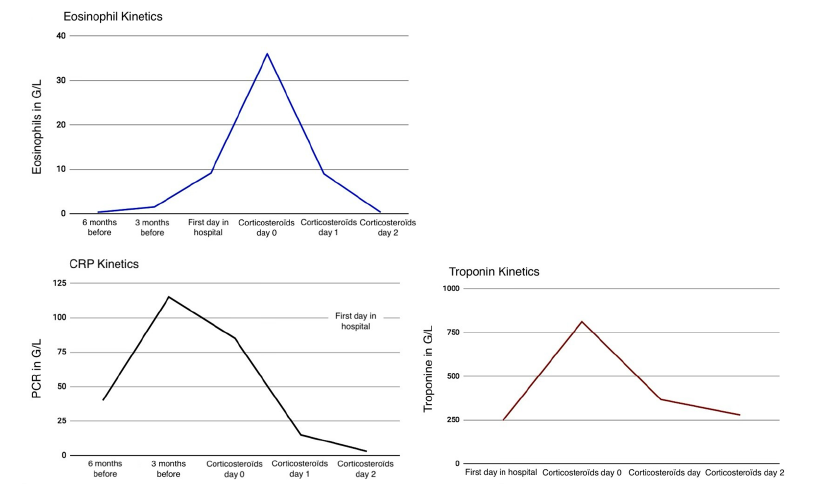
High-dose intravenous methylprednisolone (1mg/kg/day) was initiated urgently. Clinical improvement was dramatic, with resolution of chest pain within 24 hours and normalization of eosinophil count and troponin levels within 72 hours. Biological response can be appreciated by eosinophils, PCR and troponine decreases (Figure 2).
Fgure 2:Biological évolution of eosinophils, CRP and troponine before and after treatments.
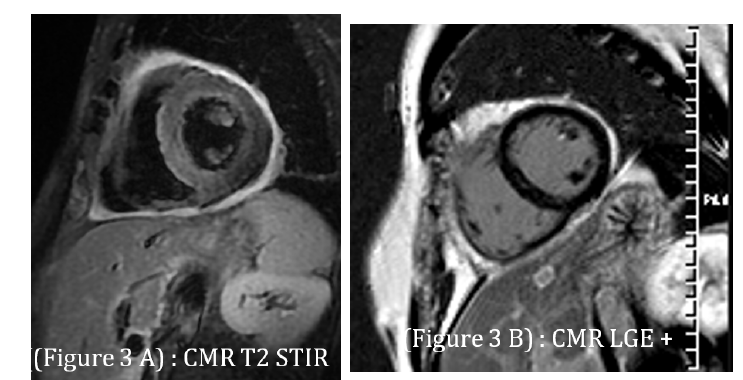
Cardiac magnetic resonance imaging (day 6) showed preserved LVEF (64%), diffuse myocardial edema on T2-weighted sequences, heterogeneous late gadolinium enhancement in the lateral wall, and pericardial thickening with enhancement, confirming acute myopericarditis (Figure 3). At 3-month follow-up, TTE demonstrated preserved LVEF and complete resolution of pericardial effusion.
Fgure 3:Cardiac magnetic resonance imaging: A. T2-weighted image demonstrating diffuse myocardial edema (hyperintensity in lateral wall); B. Late gadolinium enhancement showing patchy subepicardial involvement and pericardial thickening consistent with myopericarditis..
Discussion
EGPA is a rare systemic vasculitis, but cardiac involvement is a major prognostic factor. Myopericarditis in EGPA is mediated by eosinophil degranulation releasing toxic proteins (e.g., major basic protein), causing myocardial necrosis and subsequent Nibrosis [2].
Chest pain and gastrointestinal symptoms may delay diagnosis, as in our case where epigastric pain was initially attributed to dyspepsia. The dramatic increase in eosinophils (36G/L) and systemic features (purpura, neuropathy) were diagnostic clues.
Urgent immunosuppression is crucial, as delay may lead to irreversible endomyocardial Nibrosis or fatal arrhythmias. Cardiac MRI is the gold standard for conNirming eosinophilic myocarditis [3].
Conclusion
EGPA should be considered in asthmatic patients with unexplained hypereosinophilia and chest or epigastric pain. Rapid initiation of corticosteroid therapy can prevent irreversible cardiac damage and improve prognosis.
References
- Comarmond C, Pagnoux C, Khellaf M, Cordier JF, Hamidou M, et al. (2013) Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term followup of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum 65(1): 270-281.
- Mahr A, Moosig F, Neumann T, Szczeklik W, Taillé C, et al. (2014) Eosinophilic granulomatosis with polyangiitis (Churg- Strauss): evolutions in classification, etiopathogenesis, assessment and management. Current Opinion in Rheumatology 26(1): 16-23.
- Watanabe N, Tanabe K, Yoshinaga K (2018) Cardiac magnetic resonance imaging in eosinophilic myocarditis. Intern Med 57(6): 791-794.
© 2025 Julien Quillet. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






