- Submissions

Full Text
Associative Journal of Health Sciences
Coronary Calcification - Bone Porosity Interplay, Sex and Overweight Influence in Community-dwelling Elderly
José Ramón Lanz-Luces1*, Cristian Escobar Guzman2, Michele de Lima3 and Luís Fernando Escobar Guzman1,4
1Beneficência Portuguesa Hospital of São Paulo (BP), São Paulo, Brazil
2University of São Paulo (USP), São Paulo, Brazil
3de Lima Clinic - São Paulo, Brazil
4Cardio Geriatric Clinical Unit- Heart Institute of the University of São Paulo Medical School, São Paulo, Brazil
*Corresponding author:José Ramón Lanz-Luces, Cardiology Intensive Care Unit Research Group, Beneficência Portuguesa Hospital of São Paulo (BP), São Paulo, Brazil
Submission: March 17, 2025;Published: March 27, 2025

ISSN:2690-9707 Volume3 Issue5
Abstract
Background: Although coronary artery calcification (CAC) is osteoporosis associated there is need for
data considering the influence of overweight, sex and other factors in the interplay when analyzing bone
microarchitecture intermediate phenotypes in older adults.
Objectives: we aim to establish whether weight and sex are interrelated in a peripheral bone
microarchitecture-CAC scenario in those particular patients, considering factors that could alter calcium
homeostasis.
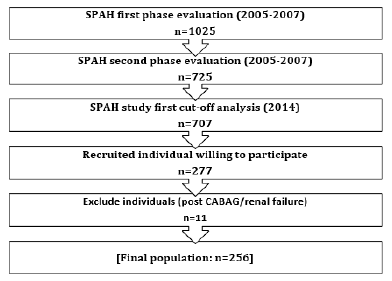
Study design: This is retrospective cross-sectional research involving 256 older adults, 168 women and
88 men, from the São Paulo Ageing & Health (SPAH) Study.
Method: The Agatston score assessed CAC and high-resolution peripheral quantitative computed
tomography (HR-pQCT) tibia and radius bone microarchitecture. A Poisson log-link regression model
was used to gauge rate ratio probability between coronary score >100, HR-pQCT variables, factors
osteoporosis-related (calcium supplementation, bisphosphonates, vitamin D, coffee, aspirin), sex and
body mass index (BMI).
Result: The population’s mean age was 79.5+4 years. One hundred and ninety-six showed BMI>25
(76.6%), with higher female prevalence (83.9% vs. 62.5%, p <0 .001). Coffee intake and medication for
bone health lacked association. In the general group, radio porosity was CAC associated (Exp(β):1.058,
CI95%:1.018-1.100, p=0.005) and aspirin (Exp(β): 1.432, CI95%:1.097-1.869). Tibia trabecular number
had a negative interrelation (Exp(β): 0.789, CI95%: 0.654-0.953, p=0.014. When divided by BMI groups,
those with BMI>25 maintained the relations (all p <0.05) however it was lost in the remaining group.
Selecting BMI>25 cases, in women only aspirin and radio porosity were related to CAC, in men were
trabecular parameters (p<0.05).
Conclusion: Coffee or medication intake used in bone metabolism lacks associations in the models
studied. The CAC-bone microarchitecture relationship is modified by weight in older adults. Furthermore,
being overweight entailed different performances for cortical and trabecular bone microarchitecture and
aspirin intake between sexes. At last, our findings shows that in older adults, sex and overweight express
differences in bone microarchitecture related to coronary calcification
Keywords:Coronary calcification; Osteoporosis; Overweight; Bone microarchitecture; Sex differences
Abbreviations: CAC: Coronary Artery Calcification; SPAH: São Paulo Ageing & Health study; HR-pQCT: High-Resolution Peripheral Quantitative Computed Tomography; BMI: Body Mass Index; DEXA: Dual-Energy X-Ray Absorptiometry; CVD: Cardiovascular Disease; AIDR 3D: Adaptive Iterative Dose Reduction 3-Dimensional Algorithm; BMD: Bone Mineral Density; WHO: World Health Organization; CT: Coronary Tomography; Tb.vBMD: Trabecular Volumetric Density; Tb.N: Number of Trabeculae; Tb.Th: Trabecular Thickness; Tb.Sp: Trabecular Separation; Ct.vBMD: Cortical Volumetric Density; Ct.Po: Cortical Porosity; CAPPesq: Ethics Committee of the University of São Paulo School of Medicine; EVOS: European Vertebral Osteoporosis Study; FAPESP: Sao Paulo State Research and Support Foundation; CNPQ: National Council of Science and Technology
Introduction
Vascular calcification, known as ectopic mineralization of blood vessels, is an active and cell-regulated process recognized as a significant risk factor in community-dwelling older adults [1]. Moreover, metabolic bone diseases such as osteoporosis have received considerable attention because they are associated with vascular calcification [2]. Although the Hunt study, which examined a large cohort (22 857 adults), found no association between bone mineral density and risk of cardiovascular disease (atrial fibrillation, myocardial infarction, ischemic cardiovascular disease, hemorrhagic stroke or heart failure) using dual-energy X-ray absorptiometry (DEXA) analysis [3], there are cross-sectional and longitudinal reports showing an association between osteoporosis and coronary calcification (CAC) [4,5]. Both entities, CAC and bone metabolism, share gene-gene interactions that influence, for example, the vitamin D metabolic process, cellular ion homeostasis, and the calcium signaling pathway [6].
Global statistics show osteoporosis and cardiovascular disease (CVD) both contribute independently to a consequential overall risk burden, and they are common age-related conditions. Some events suggest that different illness states may occur through the same pathophysiologic pathways when studying aging, smoking, physical inactivity, and elevated body mass index (BMI). For example, aged women who have menopause had a 2% risk of osteoporosis for every 10 units increase in coronary calcium score [7]. Increased cortical porosity is the main structural change responsible for osteoporotic fractures in older women and men. Imaging techniques such as high-resolution peripheral quantitative computed tomography (HR-pQCT) allow accurate 3D delineation of this bone layer and offer clinical advantages over dual-energy X-ray absorptiometry (DXA). This advantage is explained because DXA assessing osteoporosis is based on bone mineral density (BMD) and does not provide information about the bone microstructure, an issue concerning the gold standard endorsed by the World Health Organization (WHO) [8].
An adjacent scenario is obesity, an environment in which proinflammatory adipose tissue cytokines can promote the formation of atherosclerotic plaques [9,10]. It is worth noting that Class-I obesity has a more favorable prognosis compared to individuals with standard weight or underweight, a controversial paradox in cardiovascular disease [11]. Of note, the definition of obesity based on BMI could include individuals with increased lean mass and less adipose tissue, which is uncommon in older patients where sarcopenia can appear. Therefore, the complexity of these individuals is considered an emerging area in geriatric medicine [12]. In addition, some sex differences are also present in this particular population segment. Some authors observed different levels of bone biomarkers depending on sex in a population segment of 65-85 years [13].
Despite the well-documented link between obesity and cardiovascular disease, the interaction between obesity and bone metabolism is still largely unknown. Previous research studies suggest that obesity has a beneficial effect on bone density through increased muscular strain by passive load [14], but it is uncertain about the response to skeletal microarchitecture-CAC association.
Therefore, the present study aimed to investigate the hypothesis that there is an association between BMI>25 and sex influence when comparing HR-pQCT and the Agatston CAC >100 units thresh old, considering some factors that could influence bone metabolism and homeostasis in older adults.
Material and Methods
This cross-sectional analysis is based on information from the São Paulo Ageing & Health Study (SPAH) [15] and recent research that links coronary calcium concentration and bone microarchitecture [4]. People 65 years and older living in the Butantã neighborhood in the west zone of São Paulo’s made up the study’s demographic. From a medical point of view, none of the participants had cancer, severe chronic illnesses, liver disease, chronic malabsorption, or chronic diarrhea. None of the participants took protein supplements or anabolic steroids. All participants completed a standard lifestyle and health questionnaire, concerning calcium intake in the diet, family history of hip fractures, fragility, and previous fractures, history of falls in the past year, alcohol and tobacco use, physical activity, coffee consumption (>1 cup per day), and personal history of hypertension and diabetes mellitus. The subjects’ weight (in kilograms) was divided by their height (in square meters) to determine their body mass index (BMI). We used a cut-off value of 25 to define two groups (BMI>5 vs. BMI≤25).
Bone-affecting substances studied included vitamin D, calcium base phosphate binders, supplementary calcium, aspirin, and coffee. Coffee intake formed two groups, those that consume more than one cup or not, equivalent to 50ml black coffee Brazilian blend with an estimated caffeine content of 23-30mg/ml on average [16].
Evaluation of coronary calcification
Image acquisition for coronary calcification assessment was performed using a 320-detector coronary tomography (CT) system (Aquilion One ViSION, Toshiba Medical System, Japan), with cardiac synchronization using prospective triggering and a maximum temporal resolution of 125-250ms. The generated slice thickness of 0.5mm was reconstructed to a 3mm slice thickness for analysis, the X-ray tube peak voltage was set to 120kV, and the tube current was adjusted to the patient’s body size, with a range of 250-450mA. The adaptive iterative dose reduction 3-dimensional algorithm (AIDR 3D) was used for iterative reconstruction, resulting in a radiation dose between 2.6 and 4.0mSv. The method was in accordance with the Society of Cardiovascular Computed Tomography and the Society of Thoracic Radiology guidelines [17]. The CAC values were summed to calculate the Agatston score on a dedicated workstation (Aquarius Workstation, TeraRecon, Inc., San Mateo, CA). The nonenhanced scan protocol was used to analyze the calcium score evaluation. The images were evaluated by a specialist with at least 2 years of specific training who was unaware of the patient’s clinical and tomographic information.
Distal tibia and radius HR pQCT measures
The radius and tibia’s distal section were assessed, 110 slices total, or a 9.02mm long section along the axial direction, were used in the measurement. The first slice of the scan was placed at preset distances from the reference line of 9.5 and 22.5mm for the distal radius and tibia, respectively [18]. A three-dimensional HRpQCT system (XtremeCT; Scanco Medical AG) was used to measure the microarchitecture. With a resolution of 82 m (voxel size), this technology enabled the simultaneous acquisition of a stack of parallel slices. The imaging parameters employed were 1536 1536 matrix, 95mA X-ray tube current, and 60kVp effective energy.
The trabecular parameters examined were as follows: Tb.vBMD, or trabecular volumetric density (mg HA/cm3); Tb.N: or number of trabeculae (1mm, SD); Tb.Th: trabecular thickness (mm); Tb.Sp: or trabecular separation (mm); and cortical parameters, including Ct.vBMD: cortical volumetric density (mg HA/cm3); Ct.Po: cortical porosity.
To establish cortical parameters, computerized segmentation software known as “advanced cortical analysis” was utilized in addition to conventional morphological analysis [19,20].
For the density parameters at the tibia site, the HR-pQCT precision measurement from our lab’s coefficient of variation ranged from 0.25 to 1.16%. The data obtained from this study complied with the Declaration of Helsinki’s general principles, and its 64th WMA General Assembly, Fortaleza, Brazil, October 2013. The research was approved by the Ethics Committee of the University of São Paulo School of Medicine (CAPPesq #1.552.036) from a stem study [4].
Statistical analysis
The analysis was conducted using SPSS statistical software, IBM Corp. (2017). IBM SPSS Statistic for Windows, Version 25.0. Armonk, NY: IBM Corp. Continuous variables are expressed as medians with standard deviations. Depending on the Kolmogorov– Smirnov test for normality, variables were assessed in parametric or non-parametric tests. Therefore, the entire sample of continuous variables was divided by BMI, and differences were assessed using either the Mann-Whitney test or Student’s t-test, depending on the distribution pattern. A Chi-squared test was used to assess binomial variables. Variables related to HRpQCT were standardized into z-scores using the following formula: z= (observed valuesample mean)/sample standard deviation. Because, bone density structure is strongly sex influenced besides BMI, we used both variable models assessing it. The Log-link Poisson regression model determined the relation using the CAC score values, as the dependent variable, in contrast with the other factors that could interfere with bone microstructure. The model was adjusted to limit variable dispersion distribution (robust or modified method) [21].
Result
The average age of the population was 79.5+4 years. One hundred and ninety-six had an BMI >25 (76.6%), with a significantly higher proportion of women. Regarding lifestyle habits, smoking was more prevalent in the group with BMI≤25, and coffee showed a similar distribution in both groups. In lifestyle habits, smoking had elevated prevalence in the BMI >25 group, while alcohol consumption made no difference between the groups. As expected, those with metabolic syndrome, hypertension, and dyslipidemia were the majority in the BMI >25 group, (Table 1). No differences were observed between groups when analyzing medications. Renal function was significantly higher in the BMI≤25 group but did not contribute to the regression models.
Table 1:Population characteristics.
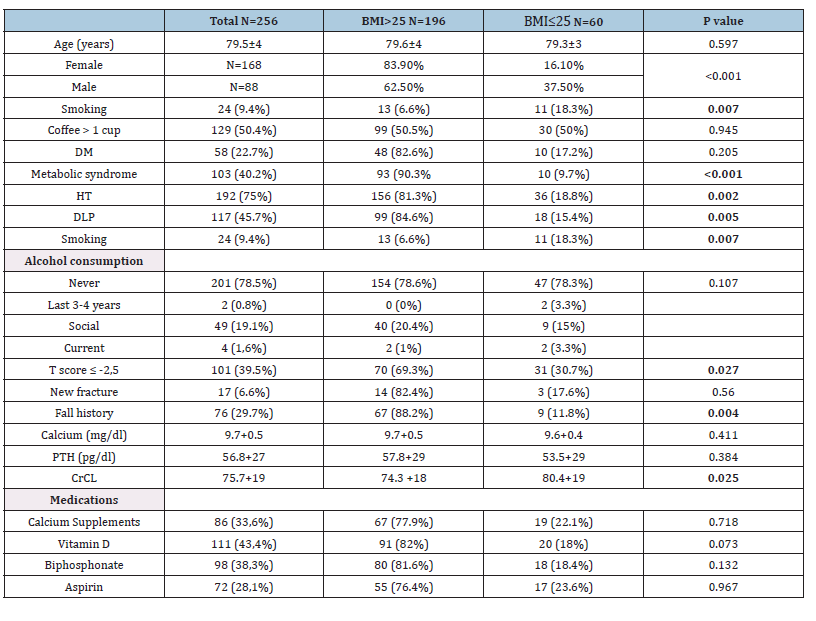
Legend: PTH: parathyroid hormone, CrCL: Creatinine Clearance.
There was a high prevalence of women in the BMI >25 group, who had more prevalence of metabolic syndrome, hypertension, and dyslipidemia as comorbidities. For bone antecedents, the BMI >25 group also presented more cases of T-score ≤2.5 and a history of falls. Otherwise, renal function was significantly less in this group. Medications that could affect bone metabolism had similar representation between groups. In terms of microarchitecture, both groups were generally homogeneous. Differences were only observed in two areas of the tibial skeleton. Individuals with a BMI >25 had significantly higher values for the volumetric bone density parameter and bone structure (Tb.vBMD, CtTh). No difference was observed in radius measurements (Table 2). Creatinine clearance showed differences between the BMI groups (Table 1) but did not contribute to the model in the univariate analysis. Calcium levels and PTH were also removed from the model. However, coffee consumption and vitamin D were retained.
Table 2:Bone microarchitecture and BMI groups.
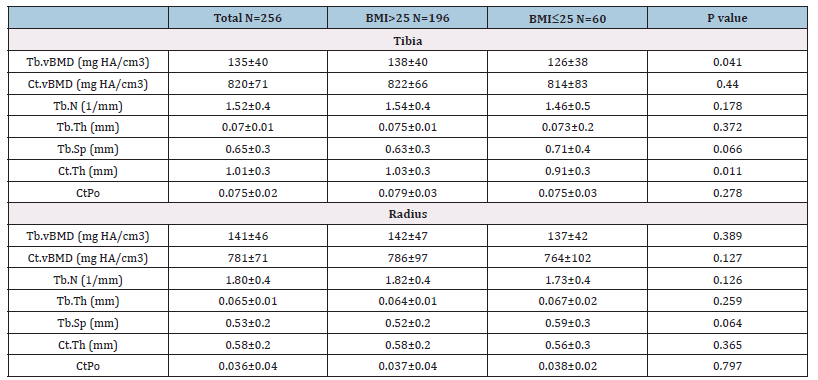
Legend: CtPo: cortical porosity, TbN: Mean trabecular number, Tb.vBMD: Trabecular bone mineral density.
Comparing bone microarchitecture with CAC, using Agatston >100 units cutoff value, in the presence of factors affecting bone metabolism, considering sex and BMI, we found no association. Note to point out aspirin had a positive association with CAC. Interestingly we observed that while radius Ct.Po was positively associated, Tibia TbN showed an inversed relationship, and radius vBMD accompanied the radius Ct.Po behavior (Table 3). When forming two groups depending on BMI, aspirin kept the association with CAC in the BMI >25 group (Table 4) and not in the opposite group (Table 5). Age and sex lacked association in both models and similar performance to medications that can alter calcium metabolism (vitamin D, bisphosphonates, and calcium supplements). Despite coffee having opposite displays depending on the BMI, in both situations, without showing any significant association. Bone microarchitecture parameters performed alike the general population for those with BMI >25, losing a link with CAC (Table 5).
Table 3:Agatston score and bone microarchitecture relationship in the general population.
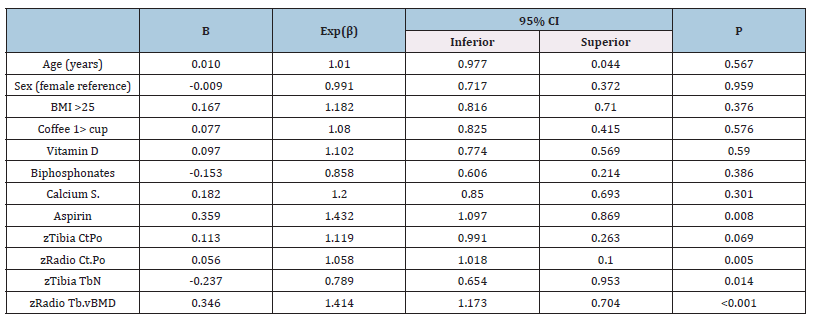
Legend:Dependent variable: Agatston score >100, Bone values are expressed in z score, CtPo: cortical porosity, TbN: Mean trabecular number (number of trabeculae per unit length, mm), Tb.vBMD: Trabecular bone mineral density.
Table 4:Agatston score and bone microarchitecture relationship in the general population.
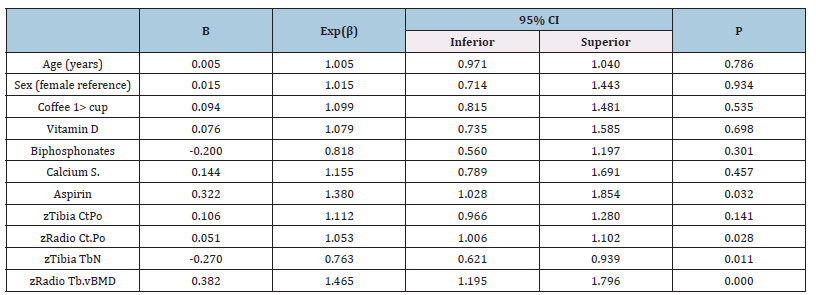
Legend:Dependent variable: Agatston score >100, Bone values are expressed in z score, CtPo: cortical porosity, TbN: Mean trabecular number (number of trabeculae per unit length, mm), Tb.vBMD: Trabecular bone mineral density.
Table 5:Agatston score and bone microarchitecture relationship in the general population.
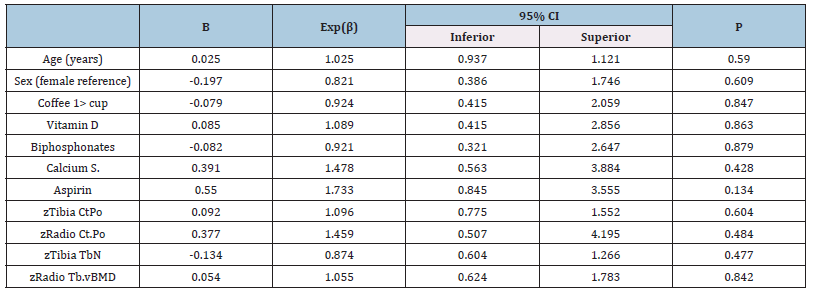
Legend:Dependent variable: Agatston score >100, Bone values are expressed in z score, CtPo: cortical porosity, TbN: Mean trabecular number (number of trabeculae per unit length, mm), Tb.vBMD: Trabecular bone mineral density.
Because their poor contribution to the model, age and medications affecting calcium metabolism were taken out the regression analysis. Only aspirin was maintained. Men’s and women’s bone cortical porosity (tibia and radius) had opposite behavior. Tibia thickness although performed similarly for both groups, it was only significant in men (Table 6).
Table 6:Calcium score, bone parameter BMI >25 and sex.
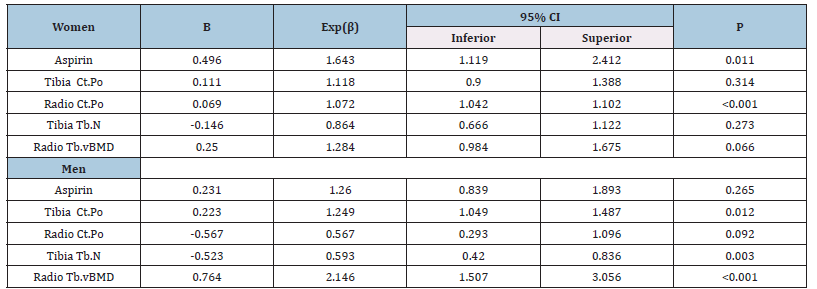
Legend:Dependent variable: Agatston score >100, Bone values are expressed in z score, CtPo: cortical porosity, TbN: Mean trabecular number (number of trabeculae per unit length, mm), Tb.vBMD: Trabecular bone mineral density.
Discussion
Instead of being essentially a passive process of calcium phosphate precipitation or adsorption in end-stage atherosclerosis, the calcification of vascular tissue is considered a highly structured process regulated by mechanism similar to those engaged in bone mineralization [22,23]. Aspects previously highlighted and covered potential molecular mechanisms interwinding skeletal bone and vascular tissue through shared osteogenic regulatory molecules, response to inflammation, and hemodynamics factors [24]. Between those factors, osteocalcin is worth emphasis, a novel hormone that can alter glucose homeostasis as well as smooth muscle cells (VSCMs), and has been reported as independently associated with coronary calcification in women [25]. Osteocalcin is a bone formation marker that originates from the skeleton and indicates the potential for osteoblasts in making bone matrix. It is a marker of bone mineralization and calcium homeostasis, recognized as a strong predictor for coronary atherosclerosis severity [26,27]. Furthermore, vitamin K-dependent-osteocalcin is related to bone metabolism and metabolic disorders, including obesity and diabetes, through some molecular proteins and hormones, including adipokines [28]. A specific hormonal aspect hormonal aspect to be acknowledged in this work is pertinent to our majority group; elderly women, where estrogens deficiency can promote osteoporosis by inhibiting osteoclast formation and bone resorptive activity due to lack of the hormone signaling expressed in osteoblast, osteoclast, and osteocytes losing target genesencoding proteins such as IL-1, insulin-like growth factor 1 (IGF1), and TGFβ [29].
Weight factor
A recent study lacked to find an association between BMI and CAC [30]. Although they evaluated a large population, key elements differ from our study. First, they had younger cases (56+11 years), 52 % of males, including a high 44% of CAC scores zero, without using the cut-off value of 100 and no factors that can affect the bone turnover, as we did. Fractures are a clinical representation of this insufficient bone mineralization. In obese adults, the risk of fracture is not the same for all skeletal sites and appears to be site-dependent: the risk for some non-vertebral fractures, such as proximal humerus, upper leg, and ankle, is higher compared to normal weight [31,32], but it is lower at vertebral sites and proximal femur [33]. This heterogeneous bone performance could partially explain the mix results between sex and the regions studied. The knowledge of previous work was just a fit for the two sites that we evaluate porosity characteristics. Besides, some sites are less affected by age. For example, the trabecular density and thickness do not vary with age at the tibia site [33]. This performance might explain why in the BMI >25 group Tb.vBMD, CtTh values were higher, showing perhaps a beneficial effect of weight in this particular site. It is worth stressing that the radius is less affected by weight. Nonetheless, it’s an excellent site to evaluate cortical porosity as indicative of osteoporosis.
Caffeine influence
In a different scenario, caffeine in a greater consumption manner (800mg) has been recently known to enhance the renal clearance of calcium up to 77% [34], and a >1 cup per day was already associated with coronary calcification [35]. The Rotterdam Coronary Calcification study included a high Agatston score cutoff value (>400) and more than 3 cups of coffee as daily intake, coffee showing beneficial odds [36]. However, those studies did not address if the person was taking other substances affecting bone turnover as was in this research. Interestingly, the RANCHO study associated coffee intake with osteoporosis, but only in women without supplemental calcium as the one of milk intake [37]. They conclude that milk offset the coffee-osteoporosis association. Therefore, let’s take a coffee always with some milk should be our recommendation. This should explain controversial reports about coffee and osteoporosis.
Substances that can affect bone turn over
Bisphosphonates, calcium and vitamin D3, denosumab, teriparatide, raloxifene, and strontium ranelate are part of the medications used to treat osteoporosis. In this study, we only obtain information for three. Those are the most accessible ones in our population. Despite that, we gathered a significative statistical valuable information. We collected information regarding calcium intake and other factors like alcohol and smoking in concordance with the European Vertebral Osteoporosis Study (EVOS) [38]. Worth noting, the questionary was design for multinational populationbased studies. Unfortunately, we did not observe any relationship with the bone parameters and the cause might be adjourn to the cross-sectional design, and small sample, thus encouraging further cohort research.
Nonsteroidal anti-inflammatory drug (aspirin)
Respecting aspirin and its interaction with bone metabolism has been elusive to be related to osteoporosis. However, epidemiological data in the study derived from the NHANES population, using dualenergy X-ray absorptiometry (DXA), found an association with several antiplatelet agents, including aspirin in osteopenia and osteoporosis, with more significance in women [39]. It is worth mentioning that the study used bone mineral density T-scores from the femur and tibia. Furthermore, a recent publication of a substudy of the ASPREE trial failed to prevent fractures in healthy older adults, indicating a potential risk for chronic low-dose use [40]. Additionally, a large study extracted from the NANHES database evaluating the use of aspirin in women and men stratified in 10 years groups from 50 till 80 years 65 found a modest benefit on bone mineral density (BMD) in the femoral neck [41]. However, a wider group from the same survey failed to find associations between BMD and low aspirin use [42]. Both studies did not report bone microarchitecture or medications that could affect bone turnover. Thanks to a more detailed view of the bone microarchitecture by HR-pQT, we observed that aspirin had a positive association with calcium score in the general population and those with BMI>25, accompanying the porosity parameters and also the BMD. These results might explain how aspirin consumption excerpts distinct bone site behavior in this population instead of those reported by DXA methods who evaluate femur neck and spine instead of radius and tibia.
Study limitations and strengths
The research was an observational, single-center study with a limited participant number. Moreover, we quantified the use of substances that affect calcium metabolism in a simple form. There is no mention of drug doses or time of its use. BMI threshold was based on the WHO definition that provides cut-off points for adults aged 25 and older (excluding pregnant and breastfeeding women); yet, it acknowledges that in individuals very old with diminished BMI. Therefore, using an age-appropriated threshold might improve classification regarding health risk. Some authors proposed the use of age stages of adulthood [43].
Finally, it is worth pointing out that we did not intend to assess nutritional status where a BMI>27 threshold some authors recommended classifying overweight in older adults because of the changes related to fat and lean mass proportion occurring in this age [44]. However, our study has the benefit of using a precise method to quantify microarchitecture changes, supporting this new concept assessing coronary calcification-bone porosity-overweight in older populations.
Conclusion
These findings support that the coronary calcificationbone architecture relationship is weight-influenced in the elderly population. Coffee or medication intake that affects bone metabolism lacks associations in the models studied. Additionally, there are differences between the sexes in porosity, trabecular measurements, and aspirin consumption, all of which are linked to coronary calcification.
Disclosures
All authors state that they have no conflicts of interest.
Role of Funding Source
This study was not sponsored by any pharmaceutical companies and is a subpopulation of the SPAH project that was supported by grants from the Sao Paulo State Research and Support Foundation (FAPESP) #03/09313-0, #04/12694-8, and #09/15346-4; the National Council of Science and Technology (CNPQ) 2013/457590- 2013-0.
Study Association
This article is a complement of a previous study and partial data of a Doctoral thesis submitted by Luis Escobar Guzman (2022), from the Cardio Geriatric Clinical Unit - Heart Institute of the University of São Paulo Medical School.
Figure 1:Geographic map and hydrographic network of Calabria.
References
- Lee SY, Chao CT, Huang JW, Huang KC (2020) Vascular calcification as an underrecognized risk factor for frailty in 1783 community‐dwelling elderly individuals. Journal of the American Heart Association 9(18): e017308.
- Escobar Guzman LF, Escobar Guzman CA, Lopes NHM (2020) Pathophysiological and genetic aspects of vascular calcification. Cardiology Research and Practice 2020: 5169069.
- Bhatta L, Cepelis A, Vikjord SA, Malmo V, Laugsand LL, et al. (2021) Bone mineral density and risk of cardiovascular disease in men and women: The HUNT study. European Journal of Epidemiology 36(11): 1169-1177.
- Guzman LFE, Lopes NHM, Torres GHF, Takayama L, de Sousa Andrade S, et al. (2022) Coronary calcification and bone microarchitecture by high-resolution peripheral quantitative computed tomography from the São Paulo Ageing and Health (SPAH) Study. Scientific Reports 12(1): 5282.
- Syu DK, Hsu SH, Yeh PC, Kuo YF, Huang YC, et al. (2022) The association between coronary artery disease and osteoporosis: a population-based longitudinal study in Taiwan. Archives of Osteoporosis 17(1): 91.
- Wicik Z, Jales Neto LH, Guzman LEF, Pavão R, Takayama L, et al. (2021) The crosstalk between bone metabolism, lncRNAs, microRNAs and mRNAs in coronary artery calcification. Genomics 113(1 Pt 2): 503-513.
- Asadi M, Razi F, Fahimfar N, Shirani S, Behzad G, et al. (2022) The association of coronary artery calcium score and osteoporosis in postmenopausal women: A cross-sectional study. Journal of Bone Metabolism 29(4): 245-254.
- Żuchowski P, Jeka D (2024) Dual-energy X-ray absorptiometry- is it the gold standard, or is bone mineral density everything? Reumatologia 62(1): 1-3.
- Kochumon S, Al Madhoun A, Al-Rashed F, Thomas R, Sindhu S, et al. (2020) Elevated adipose tissue associated IL-2 expression in obesity correlates with metabolic inflammation and insulin resistance. Scientific Reports 10(1): 16364.
- Wang C, Liu S, Kamronbek R, Ni S, Yang K, et al. (2024) Association between IL-2 Receptor and Severe Coronary Artery Calcification in Patients with Coronary Artery Disease. Reviews in Cardiovascular Medicine 25(5): 186.
- Carbone S, Canada JM, Billingsley HE, Siddiqui MS, Elagizi A, et al. (2019) Obesity paradox in cardiovascular disease: where do we stand? Vascular Health and Risk Management 15: 89-100.
- Dionyssiotis Y (2019) Sarcopenia in the elderly. European Journal of Endocrinology 15(1): 13-14.
- Choi KH, Lee JH, Lee DG. (2021) Sex-related differences in bone metabolism in osteoporosis observational study. Medicine (Baltimore) 100(21): e26153.
- Addison O, Marcus RL, LaStayo PC, Ryan AS (2014) Intermuscular fat: A review of the consequences and causes. International Journal of Endocrinology 2014: 309570.
- Domiciano DS, Machado LG, Lopes JB, Figueiredo CP, Caparbo VF, et al. (2014) Incidence and risk factors for osteoporotic vertebral fracture in low-income community-dwelling elderly: A population-based prospective cohort study in Brazil. The São Paulo Ageing & Health (SPAH) Study. Osteoporosis International 25(12): 2805-2815.
- dePaula J, Farah A (2019) Caffeine Consumption through Coffee: Content in the Beverage, Metabolism, Health Benefits and Risks. Beverages 5(2): 37.
- Hecht HS, Cronin P, Blaha MJ, Budoff MJ, Kazerooni EA, et al. (2017) 2016 SCCT/STR guidelines for coronary artery calcium scoring of noncontrast noncardiac chest CT scans: A report of the Society of Cardiovascular Computed Tomography and Society of Thoracic Radiology. Journal of Cardiovascular Computed Tomography 11(1): 74-84.
- Boutroy S, Bouxsein ML, Munoz F, Delmas PD (2005) In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90(12): 6508-6515.
- Chen W, Shi J, Qian L, Azen AP (2014) Comparison of robustness to outliers between robust Poisson models and log-binomial models when estimating relative risks for common binary outcomes: A simulation study. BMC Medical Research Methodology 14: 82.
- Doherty TM, Detrano RC (1994) Coronary arterial calcification as an active process: A new perspective on an old problem. Calcified Tissue International 54(3): 224-230.
- Torres GHF, Guzman LFE, Alvarenga JC, Lopes NHM, Pereira RMR (2019) Association of moderate/severe vertebral fractures with reduced trabecular volumetric bone density in older women and reduced areal femoral neck bone density in older men from the community: A cross-sectional study (SPAH). Maturitas 120: 61-67.
- Vattikuti R, Towler DA (2004) Osteogenic regulation of vascular calcification: An early perspective. American Journal of Physiology-Endocrinology and Metabolism 286(5): E686-E696.
- Demer L, Tintut Y (2009) Mechanisms linking osteoporosis with cardiovascular calcification. Current Osteoporosis Reports 7(2): 42-46.
- Salari P, Keshtkar A, Shirani S, Mounesan L (2017) Coronary artery calcium score and bone metabolism: A pilot study in postmenopausal women. Journal of Bone Metabolism 24: 15-21.
- Salari P, Abdollahi M (2011) A comprehensive review of the shared roles of inflammatory cytokines in osteoporosis and cardiovascular diseases as two common old people problems: Actions toward development of new drugs. International Journal of Pharmacology 7(5): 552-567.
- Bao Y, Zhou M, Lu Z, Li H, Wang Y, et al. (2011) Serum levels of osteocalcin are inversely associated with the metabolic syndrome and the severity of coronary artery disease in Chinese men. Clinical Endocrinology 75(2): 196-201.
- Al-Suhaimi EA, Al-Jafary MA (2020) Endocrine roles of vitamin K-dependent- osteocalcin in the relation between bone metabolism and metabolic disorders. Reviews in Endocrine and Metabolic Disorders 21(1): 117-125.
- Eastell R, O’Neill TW, Hofbauer LC, Langdahl B, Reid IR, et al. (2016) Postmenopausal osteoporosis. Nature Reviews Disease Primers 2: 16069.
- Altintas S, van Workum S, Kok M, Joosen IAPG, Versteylen MO, et al. (2022) BMI is not independently associated with coronary artery calcification in a large single-center CT cohort. Obesity Science and Practice 9(2): 172-178.
- Tang X, Liu G, Kang J, Hou Y, Jiang F, et al. (2013) Obesity and risk of hip fracture in adults: A meta-analysis of prospective cohort studies. PloS one 8(4): e55077.
- Compston J (2015) Obesity and fractures in postmenopausal women. Current Opinion in Rheumatology 27(4): 414-419.
- Compston JE, Watts NB, Chapurlat R, Cooper C, Boonen S, et al. (2011) Obesity is not protective against fracture in postmenopausal women: GLOW. American Journal of Medicine 124(11): 1043-1050.
- Alvarenga JC, Fuller H, Pasoto SG, Pereira RM (2017) Age-related reference curves of volumetric bone density, structure, and biomechanical parameters adjusted for weight and height in a population of healthy women: An HR-pQCT study. Osteoporosis International 28(4): 1335-1346.
- Reuter SE, Schultz HB, Ward MB, Grant CL, Paech GM, et al. (2021) The effect of high-dose. short-term caffeine intake on the renal clearance of calcium. sodium and creatinine in healthy adults. British Journal of Clinical Pharmacology 87(11): 4461-4466.
- Miller PE, Zhao D, Frazier-Wood AC, Michos ED, Averill M, et al. (2017) Associations of coffee, tea, and caffeine intake with coronary artery calcification and cardiovascular events. American Journal of Medicine 130(2): 188-197.e5.
- van Woudenbergh GJ, Vliegenthart R, van Rooij FJ, Hofman A, Oudkerk M, et al. (2008) Coffee consumption and coronary calcification: The Rotterdam Coronary Calcification Study. Arteriosclerosis Thrombosis and Vascular Biology 28(5): 1018-1023.
- Barrett-Connor E, Chang JC, Edelstein SL (1994) Coffee-associated osteoporosis offset by daily milk consumption. The Rancho Bernardo Study. JAMA 271(4): 280-283.
- O'Neill TW, Cooper C, Cannata JB, Diaz Lopez JB, Hoszowski K, et al. (1994) Reproducibility of a questionnaire on risk factors for osteoporosis in a multicentre prevalence survey: The European Vertebral Osteoporosis Study. International Journal of Epidemiology 23(3): 559-565.
- Lv H, Wang J, Zhu Y, Hu Z, Wang Z, et al. (2022) Association between osteoporosis or osteopenia and taking antiplatelet agents in general US population of NHANES. Frontiers in Endocrinology (Lausanne) 13: 945159.
- Barker AL, Morello R, Thao LTP, Seeman E, Ward SA, et al. (2022) Daily low-dose aspirin and risk of serious falls and fractures in healthy older people: A substudy of the ASPREE randomized clinical trial. JAMA Internal Medicine 182(12): 1289-1297.
- Liu H, Xiao X, Shi Q, Tang X, Tian Y (2022) Low dose aspirin associated with greater bone mineral density in older adults. Scientific Reports 12(1): 14887.
- Swed S, El-Sakka AA, Abouainain Y, Lee KY, Sawaf B, et al. (2023) NHANES cross sectional study of aspirin and fractures in the elderly. Scientific Reports 13(1): 1879.
- Babiarczyk B, Turbiarz A (2012) Body mass index in elderly people - do the reference ranges matter? 2012 Progress in Health Sciences 12(1): 58-67.
- Lima Tavares E, Martins dos Santos D, Alves Ferreira A, Garcia de Menezes MF (2015) Nutritional assessment for the elderly: modern challenges. Revista Brasileira de Geriatria e Gerontologia 18(3): 643-650.
© 2025 José Ramón Lanz-Luces. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






