- Submissions

Full Text
Advancements in Case Studies
A Case of Paediatric Neurobrucellosis with Nodular Encephalitis and Excellent Treatment Response
Preeti Joon1*, Dangeti Sowmya Sri2 and Vinod Chaudhary3
1Department of Pediatrics, Radiant Children Hospital, India
2Neuro-radiology Fellow, Department of Radiodiagnosis, AIIMS, India
3Consultant, Department of Pediatrics, Radiant Children Hospital, India
*Corresponding author:Preeti Joon, Department of Pediatrics, Radiant Children Hospital, Jodhpur, India
Submission:September 16, 2025;Published: October 17, 2025

ISSN 2639-0531Volume4 Issue4
Abstract
Introduction: Neurobrucellosis is a rare and severe complication of brucellosis, with varied and often nonspecific neuroimaging findings, making diagnosis challenging. Neuroborreliosis, caused by Brucella bacteria, is a challenging diagnosis due to its varied neurological manifestations, which often mimic other infections like tuberculosis. The primary infection route is consuming unpasteurized dairy products. It can present as chronic meningitis, intracranial hypertension, or meningoencephalitis. Diagnosis requires a high degree of suspicion, especially in cases of chronic meningitis, to prevent delays and complications. This report reviews its clinical features, diagnosis, treatment, and prognosis.
Methods: Descriptive case report.
Case details: A paediatric patient presented with acute symptoms of altered sensorium, high-grade fever lasting two days, and intermittent abnormal posturing for one day. Initial clinical suspicion strongly favored an infective etiology. MRI revealed atypical findings, including nodular lesions in the cerebrum and cerebellum exhibiting diffusion restriction, along with diffuse cerebral edema. Serological testing returned a positive Brucella IgM result. The child was successfully treated with a 45-day triple antibiotic regimen consisting of Rifampicin, Co-trimoxazole, and Ceftriaxone, supplemented with pulse methylprednisolone followed by a tapering course of corticosteroids to address neuroinflammation. Concurrent nutritional deficiencies were also managed during treatment.
Conclusion: This case highlights the importance of considering neurobrucellosis in endemic areas, even with atypical imaging presentation. A combination of serological tests and neuroimaging is crucial for diagnosis. Timely initiation of combined antibiotic and anti-inflammatory therapy can lead to an excellent clinical outcome.
Keywords:Neurobrucellosis; Encephalitis; Atypical neuroimaging; Corticosteroids; Triple regimen; Hyperhomocystenemia
Introduction
Brucella is a genus of Gram-negative bacteria responsible for one of the world’s most common zoonoses, brucellosis, which is also known as undulant fever, Mediterranean fever, or Malta fever. This systemic, animal-specific infectious disease affects multiple systems and causes more than 500,000 new human cases annually [1]. It is transmitted to humans through contact with infected animals, such as cattle or sheep, or via the consumption of uncooked meat or unpasteurized dairy products. Endemic regions include the Mediterranean basin, Central Asia, the Middle East, sub-Saharan Africa, and the Indian subcontinent.
Brucella melitensis is the most commonly isolated species, followed by Brucella abortus and Brucella suis [2]. Pediatric neurobrucellosis is rare, with a reported incidence of only 0.8%, and its diagnosis requires a high index of suspicion [3]. The associated clinical syndromes are categorized into five subtypes: acute meningoencephalitis, meningovascular involvement, central white matter demyelination, peripheral neuro-radiculopathy, and increased intracranial pressure with papilledema [4]. The diagnostic arsenal comprises serological tests like the standard agglutination test, Tube Agglutination (TA) test [also known as Wright’s Agglutination Test (WAT)], and the Rose Bengal agglutination test, alongside blood and CSF cultures [4-8]. Neuroimaging findings vary depending on the clinical presentation [9]. We describe a case of neurobrucellosis with uncommon neuroimaging findings where timely identification led to a good outcome.
Case Presentation
An 8-year-old female with a normal birth and developmental history but incomplete immunization presented with a two-day history of altered sensorium, one day of high-grade fever (105 °F), and one day of intermittent abnormal posturing. On arrival, her Glasgow Coma Scale was E3V2M5, she was febrile (105.4 °F), and had a respiratory rate of 26/min with an oxygen saturation of 96% on room air. Physical examination revealed pallor, bilateral bowing of the legs, frontal bossing, hypopigmented light golden hair, and knuckle hyperpigmentation (Figure 1). Neurologically, she was hyperventilating, had bilateral 2mm pupils reactive to light, decreased tone in all four limbs, and bilateral upgoing plantars. Abdominal examination was unremarkable. A significant history of cattle exposure and consumption of raw goat’s milk was noted. The initial impression was acute febrile encephalopathy with raised intracranial pressure, with differential diagnoses including scrub typhus, neurobrucellosis, ADEM, viral meningoencephalitis, MOGassociated disease, and tuberculous meningitis. Management was initiated with anti-ICP measures (mannitol, 3% NaCl), empirical antibiotics (vancomycin, ceftriaxone, doxycycline, azithromycin), levetiracetam, and methylprednisolone for suspected neuroinflammation.
Figure 1:Showing A) Knuckle hyperpigmentation B) Hypopigmented hair C) Genu varum.
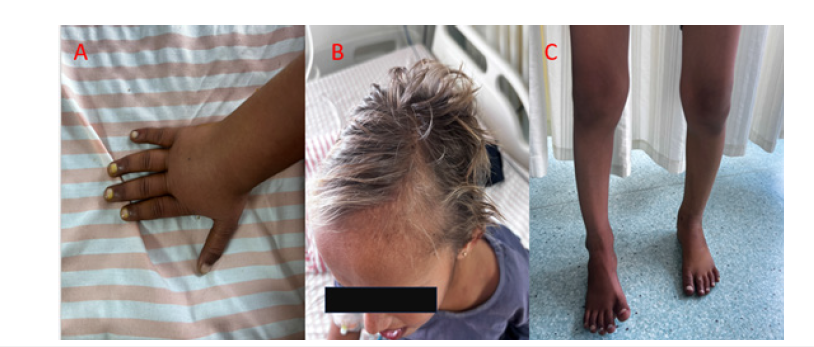
Figure 2:MRI PRE TREATMENT: Abnormal areas of nodular T2/FLAIR hyperintensities noted along the cortical and subcortical regions of bilateral occipital lobar regions (A, E) and left cerebellar hemisphere (C) with associated diffusion restriction in these areas (B, F, D). Post contrast MRI of the head, axial sections showed no significant enhancement/obvious thickening of adjacent meninges (G.H).
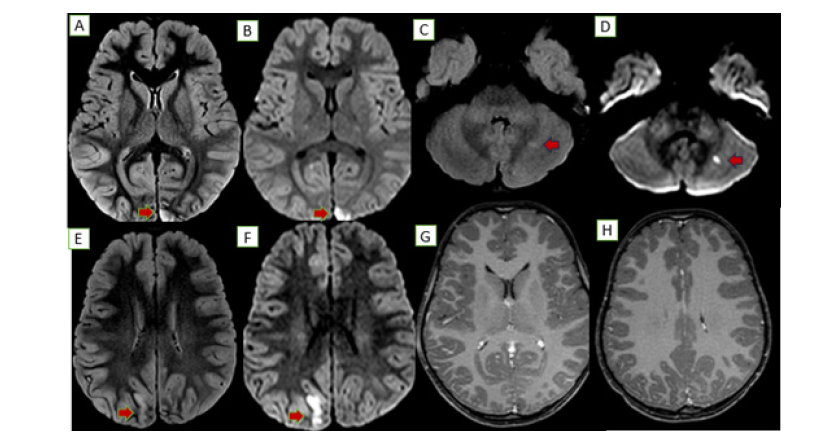
An MRI brain showed diffuse gyral edema with T2/FLAIR hyperintensities and diffusion restriction in the bilateral occipital lobes and a tiny focus in the left cerebellar hemisphere (Figure 2). Serology revealed an intermediate positive Brucella IgM and a negative scrub IgM. A CSF analysis showed 110 cells, sugar of 71mg/ dL, and protein of 34mg/dL. Further tests revealed a significantly low serum B12 (158pg/mL), elevated homocysteine (98.7μmol/L), and a high ESR (102mm/hr). The child became afebrile with initial therapy. The confirmation of Brucella exposure led to the addition of septran and rifampicin for a definitive diagnosis of neurobrucellosis. By day 4, her sensorium improved, and she was started on injectable B12 for the deficiency and Vitamin D for associated rickets. She improved gradually on a Rifampicin based triple-drug regimen for 45 days. A follow-up MRI showed residual T2/FLAIR hyperintensities with subtle atrophy but no diffusion restriction, indicating resolved acute inflammation (Figure 3).
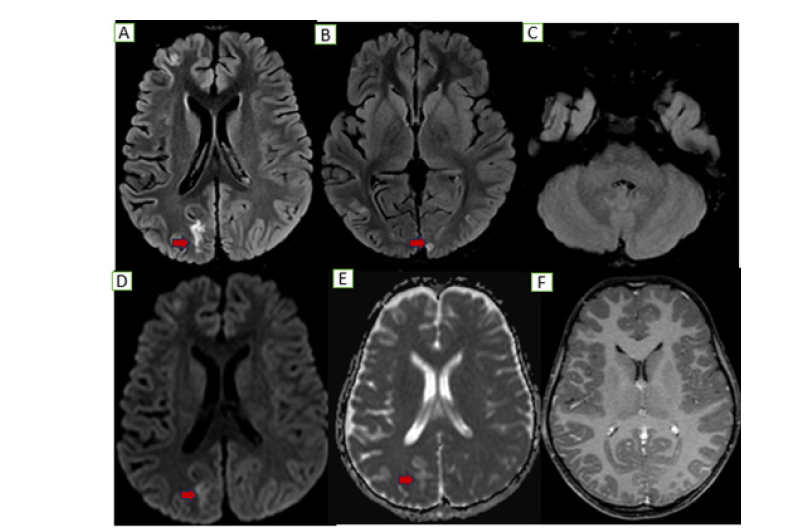
Figure 3:Follow up MRI: Axial sections show near complete resolution of previously seen nodular T2/FLAIR cortical hyperintensities along the left occipital and cerebellar hemispheric regions (B, C) with residual gliotic changes along the bilateral occipital regions (right>left A&B) without no obvious diffusion restriction (D, E) or any post contrast enhancement (F).
Discussion
The mechanism by which Brucella invades the central nervous system remains unclear. It is generally believed that after entering the bloodstream via the reticuloendothelial system, Brucella causes bacteremia and subsequently invades the meninges with a particular affinity. When host immunity declines, the bacteria begin to proliferate and invade other nervous system structures [10]. The most common clinical presentations of Neurobrucellosis (NB) include meningitis, meningoencephalitis, myelitis, brain abscess, epidural abscess, radiculitis, cranial neuritis, demyelinating disease, and vascular disease; among these, meningitis and meningoencephalitis account for approximately 50% of cases [11,12]. Our case presented with acute encephalitis syndrome with raised ICT. According to Al Sous and colleagues, imaging findings in NB can be divided into four categories: normal, inflammatory changes (evidenced by granulomas, abnormal meningeal enhancement, perivascular space enhancement, or lumbar nerve root enhancement), white matter changes, and vascular changes [13]. In our case, imaging revealed nodular lesions in the cerebrum and cerebellum with diffusion restriction, accompanied by diffuse cerebral edema.
The diagnostic armamentarium comprises serological tests such as the standard agglutination test, Tube Agglutination (TA) test [also known as Wright’s Agglutination Test (WAT)], Rose Bengal agglutination test, as well as blood and CSF cultures [4-8]. In this patient, a serum brucella IgM ELISA test was intermediately positive. In the absence of consensus guidelines regarding antibiotic choice, a combination of drugs was used. LPS (lipopolysaccharide) from gram negative bacteria is a potent neurotoxin that triggers severe brain inflammation. It does this by binding to the TLR4 receptor on microglia (the brain’s immune cells). This binding activates the NF-κB pathway, leading to the release of numerous pro-inflammatory molecules. Extremely high levels of LPS in the blood causes hyperactivation of microglia and astrocytes, resulting in neuroinflammation and impairment of neurons, synapses, and cognitive functions [14]. However, in our case LPS levels were not measured. According to a systematic review by Dhar D et al., rifampicin formed the cornerstone of therapy in the majority of cases (81.4%) [15].
We employed a triple regimen consisting of rifampicin, cotrimoxazole, and ceftriaxone for 45 days, to which the child responded excellently. For hyperhomocysteinemia secondary to vitamin B12 deficiency, the child was started on intravenous vitamin B12 and oral folic acid. Vitamin D supplementation was also administered for rickets. Additionally, in view of significant neuroinflammation, the patient received pulse methylprednisolone at 30mg/kg/day for 5 days, followed by a tapering course of oral steroids over 4-6 weeks. Given the severe neuroinflammation triggered by pathogens, pulse steroid therapy is critical. It delivers a high, immediate dose to suppress the destructive immune response, followed by a taper to ensure sustained control.
Conclusion
This case underscores the critical importance of considering neurobrucellosis as a differential diagnosis in paediatric patients presenting with acute encephalitis, particularly in regions where brucellosis is endemic. The patient’s atypical neuroimaging findings, which deviated from classic presentations, highlight the disease’s variable radiological spectrum and the necessity of integrating serological testing with clinical assessment for accurate diagnosis. The successful outcome was achieved through the timely initiation of a multidisciplinary treatment strategy. This approach combined prolonged, targeted antimicrobial therapy to eradicate the infection with adjunctive corticosteroids to mitigate significant neuroinflammation, alongside the correction of comorbid nutritional deficits. Ultimately, this case exemplifies that a high index of suspicion, comprehensive diagnostic evaluation, and a combined therapeutic regimen are paramount for the effective management of complex paediatric neurobrucellosis, leading to excellent neurological recovery.
References
- Zhuang W, Tao He, Jianyekai T, Guang He, Wang LB, et al. (2024) Neurobrucellosis: Laboratory features, clinical characteristics, antibiotic treatment, and clinical outcomes of 21 patients. BMC Infectious Diseases 24(1): 485.
- Ariza J, Bosilkovski M, Cascio A, Juan DC, Michael JC, et al. (2007) Perspectives for the treatment of brucellosis in the 21st century: The Ioannina recommendations. PLoS Med 4(12): e317.
- Habeeb YKR, Ai-Najdi AKN, Sadek SAH, Onaizi EA (1998) Paediatric neurobrucellosis: Case report and literature review. J Infect 37(1): 59-62.
- Al Deeb SM, Yaqub BA, Sharif HS, Phadke JG (1989) Neurologyclinical: Characteristics, diagnosis, and outcome. Neurology 39(4): 498-501.
- Yagupsky P (1999) Detection of brucellae in blood cultures. J Clin Microbiol 37(11): 3437-3442.
- Guven T, Ugurlu K, Ergonul O, Aysel KC, Sebnem EG, et al. (2013) Neurobrucellosis: Clinical and diagnostic features. Clin Infect Dis 56(10): 1407-1412.
- Dıaz R, Casanova A, Ariza J, Ignacio M (2011) The rose Bengal test in human brucellosis: A neglected test for the diagnosis of a neglected disease. PLoS Negl Trop Dis 5(4): e950.
- Kocman EE, Erensoy MS, Tasbakan M, Meltem Ç (2018) Comparison of standard agglutination tests, enzyme immunoassay, and coombs gel test used in laboratory diagnosis of human brucellosis. Turk J Med Sci 48(1): 62-7.
- Jiang C, Shen L, Feng Q, Wei F, Ruisheng J, et al. (2018) MRI features and categories of neurobrucellosis: A pooled review. Radiol Infect Dis 5(1): 1-6.
- Kizilkilic O, Calli C (2011) Neurobrucellosis. Neuroimaging Clin N Am 21(4): 927-37.
- Rodríguez AM, Delpino MV, Miraglia MC, Giambartolomei GH (2019) Immune mediators of pathology in neurobrucellosis: From blood to central nervous system. Neuroscience 410: 264-73.
- Karsen H, Tekin KS, Duygu F, Yapici K, Kati M (2012) Review of 17 cases of neurobrucellosis: Clinical manifestations, diagnosis, and management. Arch Iran Med 15(8): 491-494.
- Al Sous MW, Bohlega S, Al Kawi MZ, Jehad A, Donald RM (2004) Neurobrucellosis: Clinical and neuroimaging correlation. AJNR Am J Neuroradiol 25(3): 395-401.
- Vinciane K (2022) Neurological diseases function in relation to endogenous lipopolysaccharides. Clin Neurol Neurosurg 5: 160.
- Dhar D, Ravi SJ, Mahammad SM, Nagarathna S, Mahale R, et al. (2023) Pediatric neurobrucellosis: A systematic review with case report. Journal of Tropical Pediatrics 69(1): fmad004.
© 2025 Preeti Joon. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 





.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






