- Submissions

Full Text
Academic Journal of Engineering Studies
Samarium (III) Extraction
Sid Ahmed Elhabiri and Mohamed Amine Didi*
Laboratory of Separation and Purification Technologies, Algeria
*Corresponding author: Mohamed Amine Didi, Laboratory of Separation and Purification Technologies, Algeria
Submission: August 4, 2020 Published: August 15, 2021
.jpg)
ISSN:2694-4421 Volume2 Issue1
Abstract
The liquid-liquid extraction of samarium (III) from aqueous nitrate solution using D2EHPA (di-2-ethylhexyl phosphoric acid) as extractants is investigated to recover samarium (III) from aqueous solution. The effect of operating parameters, such as time, nitrate ion, aqueous phase acidity, concentration of the extractant, temperature on the samarium extraction. At pH=5.05, maximum extraction efficiency was obtained with 2 mmol L-1 D2EHPA in dichloromethane at initial concentration of Sm3+ 1 mmol L-1. D2EHPA extracts Sm3+ very rapidly. Equilibrium was reached within 15 min. The synergistic effect showed that addition of D2EHPA to TOP (Tri-iso-octyl-phosphate) extraction was obtained for the volume ratio 4.5/0.5. The thermodynamic functions like free energy (ΔG), enthalpy (ΔH) and entropy (ΔS) of extraction mechanism are discussed. By liquid-liquid extraction the removed quantity was 93.26 mg g-1. The stripping efficiency was found to be quantitative in HNO3 and HCl 1 M. The robustness of the procedure is demonstrated by the average recoveries obtained (>99.6 %) for samarium (III).
Keywords: Samarium (III); D2EHPA; Optimization; Extraction
Introduction
The recovery of heavy metals by removal from dilute aqueous system has required the development of new technique for their concentration and separation [1]. In recent years, Rare Earth Elements (REE) have been regarded as vitally important components from an industrial point of view. The major reason for this is the high application of the REE in many fields as these elements and their compounds find various commercial applications. Samarium is primarily used in the production of samarium-cobalt permanent magnets, which are used in lightweight electronic equipment’s where the size or space is a limiting factor, and where function at high temperature is of a great concern. Stable samarium titanate compounds with useful dielectric properties are suitable for coatings and in capacitors of microwave frequencies. The specific applications of samarium in different fields of technology have turned samarium into an industrial material of outstanding significance [2]. Intricately similar in their chemical properties, lanthanides pose an exigent problem in their separation. Therefore, separation of trivalent lanthanides is still a very important and serious problem. The method used for this purpose, solvent extraction is the most popular and versatile techniques [3]. This paper describes the extraction of samarium (III) by liquid-liquid using D2EHPA (di(2-ethylhexyl) phosphoric acid) diluted with dichloromethane. Solvent extraction is widely applied to processes of metal ions recovery, ranging from aqueous solutions in hydrometallurgical treatment to environmental applications. It is also considered a useful technique to increase the initial concentration of the solute, commonly used in the separation processes of analytical applications [4]. Di-2-ethylhexyl- phosphoric acid (D2EHPA) is extensively used as an extractant for the extraction of Sm(III) from aqueous solutions.2Other applications of this popular extractant include the removal of Zn(II) [5], Mn(II) [6], Fe(III)[7]. The objective of this study is to investigate the best conditions for samarium(III) extraction by D2EHPA by varying diverse parameters as shaking time, initial pH of aqueous solution, and the temperature.
Material and Method
Chemicals and reagents
Samarium (III) nitrate hexahydrate is procured from Sigma (ALDRICH), Hydrochloric acid, used for adjusting pH of samarium (III) solutions, is from Stinnes chemicals. Tri-isooctyl- phosphate (TOP) (Alfa Aesar), tri-butyl-phosphate (TBP) (Sigma Aldrich) and di (2- ethylhexyl) phosphate (D2EHPA) (Sigma Aldrich) were dissolved in dichloromethane (Sigma Aldrich) to achieve the required concentration. All other reagents such as buffer at pH = 4.0 (VWR Prolabo) and Arsenazo III (Alfa Caesar) were used for the analysis of the results by UV-Visible spectrometer (SPECORD 210 plus).
Liquid extraction and determination procedure for samarium (III): General extraction and stripping experiments were carried out by contacting equal volumes (5 mL) of the aqueous (samarium (III) at 1 mmol L-1) and organic phases (D2EHPA at 2 mmol L-1) (VAq/ VOrg=1) in reagent bottles. After equilibrium between two phases is established, the phases were separated by decantation. The removal of Sm (III) in three different extractants D2EHPA, TBP and TOP was investigated as a function of contact time between 2 and 15 minutes at initial concentration of samarium (III)=1 mmol L-1. The effect of solution pH on the equilibrium up take of samarium (III) from aqueous solution by D2EHPA was investigated between initial pH 1.3 and 5.05. Dilute nitric acid was used to adjust the pH of samarium solutions using a pH meter (model WTW, pH 3310 SET 2). Kinetic experiments were carried out by agitating 5mL of samarium (III) solution of concentration ranging from 0.2 to 5.0 mmol L-1 with 5mL of D2EHPA respectively in an Erlenmeyer flask of 10 mL at 20 ± 1 °C, pH = 5.05 and at a constant agitation speed of 1000 rpm. The effect of the ionic strength was studied with 5mL of samarium (III) solution (1 mmol L-1) and varying concentration of sodium thiosulfate from 0.1 to 0.4 mol L-1. Under constant pH and constant concentrations of and D2EHPA, samarium (III) extraction was enhanced by varying temperature in the range of 298-328 K. The concentration of Sm (III) in the aqueous phase was analyzed with a SPECORD 210 plus spectrophotometer using the method described in the literature. All procedures of the extraction liquid-liquid were carried out at room temperature 298 K and stirring rate at 1000rpm. In these experiments’ percentage extraction (%E) was determined as follows:
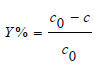 (1)
(1)
where C0 and C are the concentrations (mol L-1) of samarium ions before and after extraction, respectively.
Result and Discussion
Effect of contact time
The kinetic of extraction that describes the solute uptake rate governing the residence time of the sorption reaction is one of the important characteristics that define the efficiency of sorption. In order to establish equilibrium time for maximum uptake and to know the kinetics of extraction process; the sorption of Sm (III) for initial concentrations 1mmol L-1 by three different extractants D2EHPA, TBP and TOP is shown in Figure 1. The results in Figure 1 suggest that the % extraction increases after 10 minutes reaching the highest value in 15min. The next better extractant is D2EHPA. It is to be noted that in this case the maximum extraction is obtained in the first 10 minutes which rises slowly to reach the maximum in 15min. The extraction in TOP and TBP is almost parallel reaching maximum at 10min beyond which the extraction touches minimum at 15min Figure 1 shows the effect of time on the extraction of Sm (III) by D2EHPA, TBP and TOP. It is observed that 15min is the maximum of sorption of samarium corresponding to 49.53 % was obtained by D2EHPA. However, the maximum percent Sm(III) extraction were 9.86 and 7.7 % obtained at 10min by TOP and TBP respectively, Thereafter, becomes slower (Table 1).
Figure 1: Effect of contact time on the ion exchange of Sm(III) using D2EHPA, TBP and TOP. Initial concentration of Sm(III) 1 mmol L-1, T = 20 ± 1 ℃, stirring speed 1000rpm and initial pH 5.05.
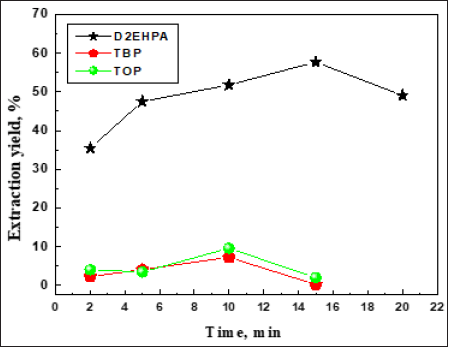
Table 1:Gibbs free energy, enthalpy, and entropy changes for Sm (III) sorption on D2EHPA.

Effect of initial pH
In the adsorption the solution pH plays an important role for controlling the high sorption capacity and selectivity of the target lanthanide ions [8-10]. This is partly because hydrogen ions themselves compete strongly with adsorbents [11]. To determine the optimum pH for the adsorption of Sm (III) ions onto D2EHPA, the percentage removal of Sm (III) ion as a function of hydrogen ion concentration was examined at an initial concentration of samarium = 1mmol L-1. The results shown in Figure 2 suggests the sorption capacity of D2EHPA to remove Sm (III) is lowest at lower pH conditions because hydrogen ions occupy most of sites on the surface of D2EHPA. However, with increasing pH of the solution, the competition by hydrogen ions is weakened because of the low hydrogen ion concentration and therefore Sm (III) ions occupy the sites more easily that results in the higher rate of removal of Sm (III) ions. The optimum pH for the maximum percentage (49.5 %) removal of Sm (III) in presence of D2EHPA is found to be pH 5.05. The mechanism was:
[Sm3+, 3 NO3-]aq + ½ [(HA)2]org ↔ [Sm.A.(NO3)2]org + (H+ + NO3-) aq (1)
Figure 2: Effect of initial pH for efficient extraction of samarium ion by D2EHPA. T = 20 ± 1 ℃, stirring speed 1000rpm, contact time 15 min for D2EHPA.
Thermodynamic studies
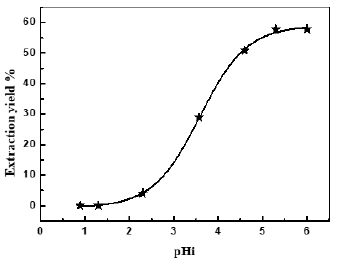
Thermodynamic parameters, such as the Gibbs energy (ΔG°), enthalpy (ΔH°), and entropy (ΔS°) changes are determined by using the following equations:
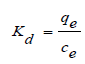
where, qe (mg g-1) was the adsorption capacity at equilibrium time and Ce was the concentration capacity at equilibrium time.
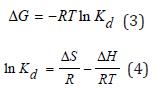
where: R (8.3145 Jmol−1 K−1) is the ideal gas constant, T (K) is the absolute temperature and Kd is the thermodynamic equilibrium constant. The values of changes of enthalpy (ΔH) and entropy (ΔS) are calculated from the slopes and intercepts of the plot of ln Kd vs. 1/T by using Eq. (4) [2]. The enthalpy of the sorption, ΔH, is a measure of the energy barrier that must be overcome by reacting molecules. The value of ΔH° for sorption of Sm (III) by D2EHPA is negative, indicating that the extraction procedure of samarium is exothermic in nature. The negative value of entropy for two extractants indicates the formation of a stable complex which makes the extraction system more ordered resulting in the decrease of entropy value. The negative value of ∆G indicates the spontaneous reaction of extraction.
Conclusion
Sorption of samarium (III) by the D2EHPA were performed. The methods were optimized using various parameters such as contact time, initial pH of aqueous solution and the temperature. D2EHPA is shown to be a good and stable extractant. It is more selective for recovery of Sm (III) from aqueous media. It should be noted that at pH 5.05, maximum extraction efficiency is obtained with 2 mmol L-1 D2EHPA in kerosene at initial concentration of Sm (III) 1 mmol L-1. D2EHPA extracts Sm (III) very rapidly. Equilibrium was reached within 15 min. Thermodynamic functions of extraction reaction were calculated and discussed. The reaction of extracting Sm(III) was found to be not spontaneous by D2EHPA extractant.
Acknowledgement
We gratefully acknowledge the DGRSDT-Algeria for their financial support.
References
- Oliveira RC, Jouannin C, Guiba E, Garcia (2011) Samarium (III) and praseodymium (III) biosorption on Sargassum sp: Batch study. Proc Biochem 46(3): 736-744.
- Wang X, Du M, Liu H (2012) Synergistic extraction study of samarium (III) from chloride medium by mixtures of bis (2,4,4-trimethylpentyl) phosphinic acid and 8-hydroxyquinoline. Sep Purif Technol 93: 48-51.
- Mandhare AM, Han SH, Anuse MA, Kolekar SS (2015) Liquid liquid anion exchange extraction studies of samarium (III) from salicylate media using high molecular weight amine. Arabian J Chem 8(4): 456-464.
- Teng TT, Yusup Y, Low LW (2012) Heavy metal ion extraction using organic solvents: an application of the equilibrium slope method.
- Pereira DD, Ferreira RSD, Mansur MB (2007) Recovery of zinc sulphate from industrial effluents by liquid-liquid extraction using D2EHPA (di-2-ethylhexyl phosphoric acid). Sep Purif Technol 53: 89-96.
- Pérez GR, Martínez JJ, Uribe SA, Martínez LA (2012) Comparative study between D2EHPA and cyanex 272 as extractants of Mn(II) from a leached liquor produced by reductive leaching of a pyrolusite ore with SO2. Engineering 4: 526-531.
- Jin Y, Ma Y, Weng Y, Jia X, Li J (2014) Solvent extraction of Fe3+ from the hydrochloric acid route phosphoric acid by D2EHPA in kerosene. J Ind Eng Chem 20(5): 3446-3452.
- Zhao X, Zhang G, Jia Q, Zhao C, Zhou W, et al. (2011) Adsorption of Cu (II), Pb (II), Co (II), Ni (II), and Cd (II) from aqueous solution by poly (aryl ether ketone) containing pendant carboxyl groups (PEK-L): equilibrium, kinetics, and thermodynamics. Chem Eng J 171(1): 152-158.
- Awual R, Kobayashi T, Miyazaki Y, Motokawa R, Shiwaku H, et al. (2013) Selective lanthanide sorption and mechanism using novel hybrid lewis base (N-methyl-N-phenyl-1,10-phenanthroline-2-carboxamide) ligand modified adsorbent. J Hazard Mater 252(253): 313-320.
- Igberase E, Osifo P, Ofomaja A (2014) The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueoussolution: Equilibrium, kinetic and desorption studies. J Environ Chem Eng 2(1): 362-369.
- Li D, Chang X, Hu Z, Wang Q, Li R (2011) Samarium (III) adsorption on bentonite modified with N-(2-hydroxyethyl) ethylenediamine. Talanta 83(5): 1742-1747.
© 2021 Mohamed Amine Didi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






