- Submissions

Full Text
Academic Journal of Engineering Studies
Cloud Point Extraction of Toxic Red Bemacid Dye by Ionic Liquids and Nonionic Surfactant System and Factorial Design
Semmoud R and Didi MA*
Department of Chemistry, Algeria
*Corresponding author: Didi MA, Department of Chemistry, Algeria
Submission: April 24, 2020 Published: September 15, 2020
.jpg)
ISSN:2694-4421 Volume1 Issue4
Abstract
The present study investigates the effects of additives on the cloud point temperature of Triton X-100 as a non-ionic surfactant in aqueous solutions (% of Triton X-100,% of salts (NaCl, Na2S2O3, Na2SO4, KI, KBr, KNO3), and concentrations of synthesized ionic liquids (Thiocyanate trioctylmethyl ammonium (LI1), Hydrogenophosphatetrioctylmethyl ammonium (LI2)). The parametric study based on the experimental design methodology by means of 24 factorial design, allows us to predict the effect of the main parameters pH (4.0-8.0), percent mass of Triton X-100 (2-10%), Na2SO4 (8-10%) and Red Bemacid dye concentration (10-100ppm) with 100ppm of synthetic ionic liquids on the extraction efficiency of the dye.The extraction using the CPE process (Cloud Point Extraction) of Red Bemacid showed to be efficient, reaching values of more than 100% dye recovery in optimum conditions using these two ionic liquids.
Keywords: Red bemaciddye; Ionic liquids; Non-ionic surfactant; Additives; Cloud point extraction; Factorial design
Introduction
Several methods for the extraction of dyes were used as coagulation, membrane process, adsorption etc.., but in the last decade, a growing interest in the use of cloud point extraction (CPE) appears because this method offer some advantages, especially elevated extraction efficiency, selectivity, experimental convenience, low cost, easily waste disposal and the use of non-toxic and less hazardous reagents [1-3].CPE is a separation and preconcentration procedure that has been widely applied for the extraction of various types (organic and inorganic) solutes [4]. The physicochemical properties of the nonionic surfactants vary in the presence of other surfactants, electrolytes, other organic or inorganic additives. It is possible to control the temperature of the disorder by varying the composition and the quantity of introduced substances [5].Textile processing industries consume huge amount of water during dyeing and finishing processes. The presence of dyes in textile wastewater inhibits photosynthetic process in water bodies and also generates toxicity to aquatic organisms and humans [6]. These effluents represent more than 50% of the world’s production of dyestuff. It is estimated that 10-20% of the initial quantities are lost during dyeing operations and are rejected without prior treatment [7].Molecular organic solvents are the most commonly used solvents in the liquid-liquid extraction treatment processes of solutes contained in an aqueous phase. Generally, in such a system, an extractant is added to the solvent for extracting the metal cation or dye in the organic phase. These extraction systems use large volumes of organic solvents, which are, unlike ionic liquids, volatile. The extraction efficiency and selectivity of some extraction systems using ionic liquids were sometimes superior to the systems using organic solvents [8].
The present work is focused on the optimization of CPE by following factorial model 24. This model was studied using variables: pH (4-8), Triton X-100 (2-10% w), Na2SO4 (8- 10% w) and the concentration of Red Bemacid (10-100ppm), in the presence of Thiocyanate trioctylmethyl ammonium (LI1) and Hydrogenophosphate- trioctylmethyl ammonium (LI2) ionic liquids (100ppm).The dependencies of the experimental conditions, such as the concentrations of Triton X-100, ionic liquids, and toxic dye, ionic strength, on the cloud point behavior, were studied.
Experimental Procedure
Reagents
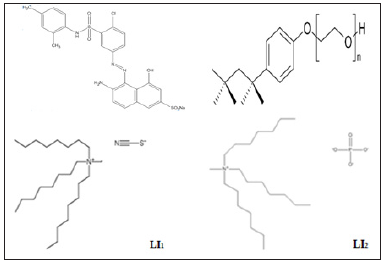
Sodium(E)-6-amino-5-((4-chloro-3-(N-(2,4-dimethylphenyl)sulfamoyl)phenyl)diazinyl)-4 hydroxynaphthalene-2-sulfonate (Red Bemacid) was obtained from the textile industry (Soitex-Algeria) (scheme 1). Triton X-100 (phenyl ether of p-octylpolyethylene glycol, with HLB=13.5 and critical micelle concentration CMC is 3.0×10-4 M at 25°C), KNO3, NH4SCN, NaH2PO4.2H2O and C3H6O were purchased from Fluka. NaCl, Na2S2O3.5H2O were purchased from Carlo Erba. KI, KBr, Na2SO4, H2SO4, Tertabutyl phosphonium p-toluenesulfonate, Trihexyl tetradecyl phosphonium bromide, Aliquat 336 and Ethanol were purchased from Sigma Aldrich. The pH values of the solutions were adjusted by adding sulfuric acid (H2SO4) and sodium hydroxide (NaOH) as appropriate (Sigma-Aldrich). Sodium sulfate was used to lower the cloud temperature and to facilitate extraction at 25°C.
Figure 1: Structure of dye red bemacid, triton X-100, ionic liquids LI1 and LI2.
Apparatus
Weighs are made with an electronic analytical balance type Carat Series OHAUS Item: PAJ1003. pH measurements were performed on a HANNA Instruments HI 2210 potentiometer. Mechanical stirrer Lab Tech E 4565/FNR/2013 hot plate was used. The Kikawerke TC-2 hot plate stirrer with a standard thermocouple was used for the temperature experiments. Dosing of the dye was carried out using a UV/Visible spectrophotometer, type SPECORD 210/Plus.
Ionic Liquids Preparation
Synthesis and Characterization of thiocyanate trioctylmethyl ammonium (R3CH3N+SCN-) (LI1)
The synthesis reaction was carried out by the addition of NH4SCN dissolved in ethanol to the quaternary ammonium salt (Aliquat336), according to the following reaction (1) and synthesis process:
R3CH3N+ Cl-+NH4SCN→R3CH3N+SCN-+NH4Cl(R=-C8H17) (1)
20mmol (1.520g) of NH4SCN in ethanol is added dropwise to 20mmol (8.083g) of Aliquat 336 dissolved in hot ethanol. The mixture is left under magnetic stirring for 2 hours at room temperature. After decantation and filtration under vacuum, the solvent was evaporated on a rotary evaporator. Washing was performed twice with 10mL of acetone followed by evaporation. Our synthetic product is a viscous yellow-brown liquid, the yield being 75%.
Synthesis and Characterization of hydrogenophosphatetrioctylmethyl ammonium (R3CH3N+H2PO4-)(LI2)
The synthesis reaction was carried out by the addition of NaH2PO4.2H2O dissolved in distilled water on the quaternary ammonium salt (Aliquat 336), according to the following reaction (2) and synthesis process:
R3CH3N+Cl- + NaH2PO4→ R3CH3N+H2PO4-+NaCl(R=-C8H17) (2)
20mmol (3.120g) of NaH2PO4.2H2O in distilled water is added dropwise to 20mmol (8.083g) of Aliquat 336 dissolved in hot ethanol. The mixture is left under magnetic stirring for 2 hours at room temperature. After decantation and filtration under vacuum, the solvent was evaporated on a rotary evaporator. Washing was performed twice with 10 mL of acetone followed by evaporation.Elimination of chloride ions was verified by the formation test of AgCl precipitate using a solution of AgNO3. The synthetic product is a viscous yellow liquid with a yield of 78%.
Determination of cloud point temperature of Triton X-100
The cloud point study involves determining the influence of a number of key parameters on the cloud point temperature of the nonionic surfactant used, which is Triton X-100. These parameters are:
The amount of Triton X-100 used: 1-10% (w/w).
[Ionic liquid]=50-100ppm.
Ionic strength: Effect of different salts 1-10% (w/w).
To do so, we have studied the effect of these parameters one by one, which brings us to examine the Triton X-100/H2O/key parameter systems. Then we plotted the binary temperature diagram of the cloud point according to the key parameter. The following figure describes the different steps in the study of cloud point variation.
General extraction procedure of Red Bemacid Dye
Extraction of Red Bemacid dye in an aqueous medium using the cloud point technique (Figure 1) was carried out in 10mL graduated tubes in which the nonionic surfactant (Triton X-100) was mixed with the extracting agent (C8H17CH3N+SCN-, C8H17CH3N+H2PO4-as ionic liquids) and the salt (sodium sulfate). The mixture was stirred and left to stand in rest for 24 hours at room temperature. After this time, the coacervate phase was separated from the dilute phase. The dye present in the dilute phase is then analyzed by UV-visible spectrophotometry.
Figure 1: Procedure adopted for the study of the extraction of dye by CPE.

Results and Discussion
Characterization
IRTF(LI1): ν (cm-1)=2929 (CH3,s), 2054(C≡N,s), 731(CH2)7, 1467(CH2& CH3), 1218(N-C, w), 588 (C-S)
IRTF (LI2): ν (cm-1)=2929 (CH3, s), 2343(P-OH, L), 1305 (P=O, s L), 729(CH2)7, 1465 (CH2& CH3), 1100 (N-C, s)
s, strong; L, large.
Cloud point study
The effect of surfactant and additives concentrations on the cloud-points was analyzed in this study.The influence of various additives at different concentrations on the Triton X-100 was presented below. When the low-temperature surface activity of surfactants needs to be increased, the addition of electrolytes is helpful [9].
Effect of Triton X-100 on cloud point
Long heating of Triton X-100 solutions to different amounts in 20mL graduated test tubes placed in a thermostatic bath determined the relative cloud point. The temperature of the first appearance of turbidity was taken as the cloud point temperature.Figure 2 shows that the cloud temperature (tcp) was 63°C, which corresponds to the literature [10].The dependence of tcp on the length of the oxyethylene chains (the number of oxyethylene groups-n) in molecules, is well-described by the Equ. (1):
tcp = b.ln(n-n0) (1)
Figure 2: Influence of the nonionic surfactant (Triton X-100) on the cloud point temperature.

where b is the coefficient of proportionality, n0=6. The constant, n0, is equal to the minimum number of oxyethylene groups in water-soluble molecules [5].Our non-ionic surfactant Triton X-100 (n-n0=4) corresponds to 65°C, which is in agreement with our result.The number of ethylene oxide groups and the length of the hydrophobic chain of the nonionic surfactant have a great influence on the turbidity of the surfactant solutions. The increase in the number of ethylene oxide chains in polyethoxylated alcohols tends to raise the temperature because of the notable solubility of the surfactant in water by interaction with the ethylene oxide groups [11].When the temperature increases, there is also an increasing in the entropy, which causes the dehydration of polyoxyethylene chains and destroys the water molecules layer. From this point, the weak Van der Waals forces among the molecules make important contributions to the micellar agglomerate formation and, consequent, the phase separation occurs [12].
Influence of ionic liquids on the cloud point
The use of ionic liquids, characterized by their wide range, has various advantages such as environmental respect [13,14].They receive a lot of attention due to of their unique properties: low melting point, negligible vapor pressure..., therefore considered as a solvent in many applications: synthesis, electrochemistry, and separation. The control of their hydrophobicity or their hydrophilicity, by changing their anions or cations, makes them very good additives [15].Extraction with ionic liquids was more effective than with chloroform [14]. Therefore, the selection of the carrier is a decisive criterion. Indeed, the extractant agent forms complexes with the solute which must be soluble in the organic phase and insoluble in the aqueous phase.The modification of the physicochemical properties of the aqueous surfactant solutions favorably by the addition of the ionic liquid has received a lot of attention [16]. Thus, micelle-surfactant and cation/anion interactions of ionic liquids in aqueous solutions have been extensively studied.
The sample solutions used for the cloud point determination were prepared by directly mixing 2% Triton X-100 and different concentrations of the ionic liquid in graduated tubes with a total volume of 10mL, then homogenized and placed in a bath thermostated by varying the temperature of 5°C every 30min. The temperature increases sharply with the increasing in ionic liquid concentrations. In the range [0-500ppm], the increasing varies from 63 °C to90 °C(Figure 3).The study of the structure effect on the cloud point was studied for ionic liquids containing ammonium cations. The hydrophobicity deficiency leaves the ionic liquid close to the micelle-water interface while preventing penetration into the Triton X-100 micelle and replacement of the nonionic surfactant monomers [17,18].
Critical micellar concentration (CMC), micelle size, aggregation numbers are the key physicochemical properties of aqueous surfactant solutions [16]. Aggregate size is significantly affected by hydrophobic and hydrophilic ionic liquids while affecting the cloud point [15,16].Figure 3 indicates that the cloud point of Triton X-100 increased by the addition of the ionic liquid. It is known that turbidity is obtained around the CMC so that its addition can alter/modify favorably the physicochemical properties of such systems [19].In previous studies, the cation interaction of the ionic liquid with a long alkyl chain can align with the surfactant molecules in the micelles. Zhang et al reported that the formation of mixed micelles in aqueous solutions with Triton X-100 is governed by hydrogen bonds and hydrophobic interactions [15]. Thus, the electrostatic repulsion between the oxyethylene groups of Triton X-100 increases due to the permeation of the cation resulting in an increasing in the CMC and consequently the cloud temperature [16].
Figure 3: Influence of ionic liquids on the cloud point temperature of Triton X-100.
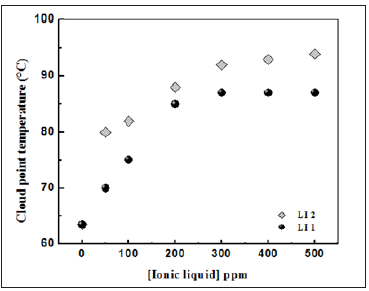
We believe that there should be interactions between hydrophobic anions and micelles, although the anion does not contain an alkyl chain. The more hydrophobic it is, the more it will interact with micelles. Ionic liquids with a hydrophobic anion do not influence as obviously on CMC and aggregation number as hydrophilic with a long alkyl chain, but obviously, affects the aggregate size and cloud point. In summary, the ionic liquid increases significantly the cloud point due to the existence of important interactions between the surfactant and an ionic liquid. Furthermore, the incorporation of ionic liquid into the non-ionic micelles causes electrostatic repulsion between the micelles, thus hindering the coacervate phase formation and raising the cloud point [20].
Influence of different salts on Triton X-100 disorder
In this extraction process, it is desirable that the cloud point (CP) of the surfactant solutions is low. Its reduction differs from one salt to another probably because of the change in the solvent property of the water due to the effects of ions.Inorganic salts can improve or reduce the cloud point by acting as salting agents [9]. Some behave like breakers and increase the CP while others are manufacturers of water structures and damage CP [21].The release electrolyte decreases the cloud point while that of salting increases it. The latter usually breaks the structure of the water improving its solvation capacity, thus increasing the solubility. A presence of structure-breaking ions can clog the self-aggregation of water molecules increasing the extent of hydrogen bond formation between water molecules and ether groups in nonionic surfactants. On the contrary, the release electrolyte associates the water molecules, thus reducing the solubility of the surfactant in the water to reduce the cloud point. Anions of electrolytes appear to be more effective at lowering cloud point than cations because of their ability to form the water structure, especially with large polyatomic anions. The overall impact of the electrolyte is the summation effect of anions and cations. According to various measurements of the effects of ions on the water structure, cations such as Li+, Na+, K+, NH4+, Ca2+, Mg2+, and anions, such as F-, SO42-, CO32-, and PO43- are structuring ions, whereas Cl-, Br-, I-, And NO3- are ions that break the structure [9].
Therefore, the case study of Triton X-100, in the presence of additives, is a common practice preceding their application in various industrial processes. The monitoring of the variations of cloud points as a function of different mass percentages of salts informs us of the influence of salinity on the cloud point (Figure 4). The low and close percentages by weight of various added salts (NaCl, Na2S2O3, Na2SO4, KBr, KI, and KNO3) do not affect CP. While the latter decreases for higher percentages except for potassium salts where ancestry is noteworthy.The different effects of their ions on the modification of the solvent property of water could be at the origin of the results of the salts. Sodium sulfate has the most negative effect. Thus, the addition of a small amount of this salt can reduce the cloud point to less than 20°C[22].
Figure 4: Influence of different salts on the cloud point of Triton X-100.
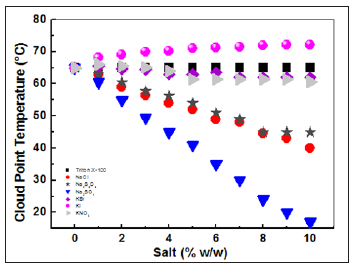
Usually, the inorganic salt anions influence the cloudiness behavior of the nonionic surfactant more than the cations. The most important effect is noted with the SO42- anion compared with the Cl- anion, which follows the Hofmeister series [23,24]. The addition of these salts gives salting out and causes a reduction of the cloud point of the nonionic surfactant because reducing the solvent property of the water for the surfactant and inducing the dehydration of the micelles. Therefore, the aggregation number and the hydrophobic environment of the micelle are increased with the salt concentration. The divalent Na2SO4 salts are more competent on the monovalent NaCl in terms of release [4]. The SO42- ions are more effective than the phase separation at 45°C, with a percentage of Na2SO4 at 4% and at the same temperature, have to be doubled the amount of NaCl.According to Figure 4, it is clear that Na2SO4 lowers CP at room temperature following dehydration of the ethylene oxide bonds by weakening the hydrogen bonding between the water molecules and the polar head of the surfactant due to solvation ions [13]. This lowering is called salting out [9,19]. The release phenomenon is mainly due to the anion of the electrolyte [25].
It is noticeable that the effect of electrolytes on the cloud point temperature decreases with increasing number of oxyethylene groups. And, at the same time, the phenomenon obeys the arrangement of anions in the lyotropic Hofmeister’s series. In terms of the degree of influence on the cloud point temperature, the anions are arranged in the series: SO42->CO32->F->Cl->I-. The action of divalent ions on tcp is much greater than that of monovalent ions [5,26].The cloud temperature remains practically insensitive to the Br-, I- and NO3- anions: Salting-in phenomenon. This is because the hydrotropic electrolyte increases the cloud point due to its water eliminating property.The formation of hydrogen bonds between the polar part of the surfactant and the water of hydration molecules is promoted by increasing the solubility of the surfactant.In summary, the purpose of adding salt with a high salt effect such as Na2SO4 is to have low-temperature phase separation. This can contribute to the reduction of energy costs for a CPE on an industrial scale [27].
Parametric study of the extraction of Red Bemacid with these two synthesized ionic liquids (C8H17CH3N+SCN-, C8H17CH3N+H2PO4-) using an experimental design
The use of the factorial model gives a global and multidimensional view of the process [28]. Among the most important advantages of this technique is that it can give a large amount of information about the process by judiciously selecting a limited number of experiments. Experimental plans make it possible to better organize the tests that accompany scientific research or industrial studies [29]. They are applicable to many disciplines and all industries from the moment we look for the link between a quantity of interest Y (the yield in our case) and variables X (pH, concentrations, % weight, ... ) (Table 1). This study allows us to observe the effects of the following parameters: the concentration of the ionic liquid (C8H17CH3N+SCN-, C8H17CH3N+H2PO4-), the initial pH, % w of Triton X-100, % w of Na2SO4 and the concentration of Red Bemacid. Thus, we obtain a mathematical model that summarizes our experimental study. The dye extraction yields in each experiment are shown in Table 2.
Table 1: The 4 factors and their values.

Table 2: Experimental data.

Figure 5 shows the cloud point extraction of Red Bemacid dye.In this investigation for quantification of the effects of four variables on the Red Bemacid removal, a two levels factorial design (low and high) of experiments was adopted.For the current experiment, measurements of volume, weight, and dye analysis are the main causes of errors. For this purpose, three additional central point attempts (0,0,0) are required to estimate the average error in the value of each coefficient, based on the random variance. Calculations are summarized in Table 3.Thus, with 95% confidence (α=0.05), and for two variances (three attempts at the central point), the value of tv-α/2 as 2.92. The relation of the extraction efficiency of Red Bemacid Dye by the ionic liquid, as a function of the reduced variables X1, X2, X3 and X4 is given by equations(2) LI1 (3) LI2:
Y=76,71-1,34X1+6,74X2+7,04X3-0,62X4+1,61X1X2+1,79X1X3-6,29X2X3+1,28X3X4-2,07X1X2X3+0,78X1X2X4 Equ(2)
Y=92,61+0,09X1+4,05X2-2,20X3-4,69X4+0,48X1X2+0,42X1X3+2,25X2X3+2,53X2X4+1,28X3X4+1,00X1X2X3 +3,78X2X3X4 +0,48X1X3X4+0,53X1X2X4-1,05X1X2X3X4 Equ (3)
Table 3: Model adequacy tests and analysis of variance.

Figure 5: Cloud Point Extraction of Red Bemacid dye.

Factors influencing the extent of extraction
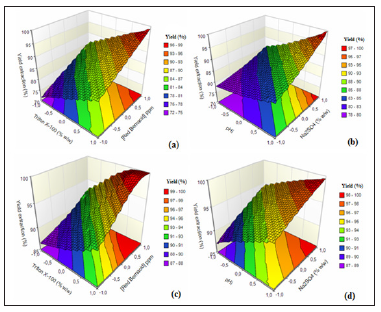
Figure 6 shows the surface response graphs of Red Bemacid extraction and the effects of the different factors on its efficiency
Figure 6: Response surfaces for extraction of red Bemacid by the two ionic liquids (a,b) with Thiocyanate trioctylmethyl ammonium. (c,d) with Hydrogenophosphatetrioctyl methyl ammonium.
Effect of surfactant concentration on extraction
Among the surfactants, Triton X-100 was chosen for its higher extraction efficiency as well as for its appropriate cloud point temperature relative to the other surfactants tested. Figure 6a-6c describes the effect of Triton X-100 concentration on the extraction efficiency of Red Bemacid dye. As observed at a fixed temperature of 25 °C and a fixed ionic liquid concentration of 100ppm, dye extraction efficiency increases with surfactant concentration.The concentration of micelles increases with the concentration of surfactant, followed by greater solubilization of the dyes in the micelles. As a result, dye extraction efficiency increases with surfactant concentration. The optimal surfactant concentration of 10% by weight of Triton X-100 was chosen for the formation of hydrogen bonds between water molecules and ether groups in nonionic surfactants. Quantitative removal of Red Bemacid dye (>85%-90%) with LI1, LI2 respectively, was achieved using at 10% w Triton X-100 [30,31].
Effect of pH on yield extraction
For this study, the effect of a pH range of 4 to 8 on Red Bemacid CPE using factorial curves was studied. The effects of the pH of the solution on the extent of extractions of the Red Bemacid are shown in Figure 6b-6d using the Triton X-100-LI1 and Triton X-100-LI2systems respectively. The extraction efficiency had a lower acid pH and increased with pH. In the event of a decrease in pH, the recovery of Red Bemacid decreases and, at high pH, the extraction efficiency of the dye becomes high (85% with LI1 and 95% with LI2). At lower pH, the dye studied was protonated and their ionic characteristics increased, resulting in less solubilization of the dye in the hydrophobic micelles. On the contrary, at high pH, the dyes were deprotonated, and their behavior resembled a hydrophobic molecule and solubilized easily in the micelles. As a result, the solubility of the dyes is greater at the basic pH, resulting in an increasing of dye extraction of 78-85% with LI1 and 87-95% with LI2 from Acid pH at Basic pH [20,32].
Effect of dye concentration on extraction
It is obviously observed that the extraction efficiency of Red Bemacid dye increases with the dye concentration for both cases (Figure 6a-6c). It is well known that micelle concentration increases with surfactant concentration, followed by greater solubilization of dyes in micelles. It can be said that when the concentration of the dye and its solubility in surfactant micelles vary in the same direction [33]. Therefore, the extraction efficiency of Red Bemacid is ascending. In our study, all (100%) Red Bemacid dye was eliminated by working at the maximum concentration (100ppm).
Effect of ionic strength in the extraction
The cloud point of micellar solutions can be controlled by adding salts, alcohols, nonionic surfactants and some organic compounds (salting-out) [34]. In the CPE technique, the addition of salt to the sample solution facilitates phase separation and increases the mass transfer of the analyte from the aqueous phase to the surfactant-rich phase. In addition, the presence of small amounts of inorganic salts causes a decreaseing in the cloud point temperature. Therefore, it is essential to take into account the side effects of the electrolyte, namely the release [35]. On the basis of these reasons, Na2SO4 salt was chosen and its effect on the extraction process was examined. From the results (Figure 6b-6d), the highest recovery of Red Bemacid was obtained with a Na2SO4 concentration of 10%). This effect could be explained by the additional surface charge when the concentration of Na2SO4 is very high, which modifies the molecular structure of the surfactant and, consequently, the micelle formation process [34]. Therefore, a Na2SO4 concentration of 10% (weight) was adopted as the optimum value.
Conclusion
The results obtained in this study indicate that the sodium salts decrease the CP and contribute to the increasing of the hydrodynamic size of the micelle in the order Na2SO4>Na2S2O3>NaCl.It allows to obtain significant extraction yields without heating solution and to work at room temperature. It can be carried out using Triton X-100 as a surfactant, trioctyl methylammonium thiocyanate and triphenyl methylammonium hydrogeno phosphate as chelating extractants and sodium sulfate. From the experimental results, quantitative removal of Red Bemacid dye 100% efficiency to 100ppm of the dye was achieved using 10% by weight Triton X-100, 10% by weight Na2SO4 in basic medium (pH=8) to obtain the optimal analytical signal associated with the highest possible extraction efficiency.The results show that the dye can be removed significantly in a single CPE extraction under optimal conditions and the polynomial models developed here can provide a valuable basis for industrial scale applications.
Acknowledgement
We gratefully acknowledge the DGRSDT and ATRST-Algeria for their financial support.
References
- Guo C, Ren Y, Zhou P, Shao J, Yang X, et al. (2012) Toward a quantitative model and prediction of the cloud point of normal nonionic surfactants and novel Gemini surfactants with heuristic method and gaussian process. J Disper Sci Technol 33(10): 1401-1410.
- Kuo W S, Chen W Y (2012) Int J Photoenergy 1-7.
- Amorim KP, Andrade LS (2017) Development and application of a cloud point method for the extraction of natural estrogens E1 and E2 from urine samples and their simultaneous determination by HPLC-EC using a BDD electrode. Anal Methods 9(10): 1627-1633.
- Pan T, Xu M, Chen X, Sun G, Guo J (2013) Cloud point extraction of four triphenylmethane dyes by triton x-114 as nonionic surfactant. Sep Sci Technol 48(7): 1040-1048.
- Arkhipov VP, Filippov A (2018) The cloud point of aqueous solutions of ethoxylated monoalkylphenols in the individual state and in the presence of electrolytes. J Disper Sci Technol 39(10): 1442-1446.
- Wanga L, Zhanga J, Wanga A (2008) Removal of methylene blue from aqueous solution using chitosan-g-poly (acrylic acid)/montmorillonite superadsorbent nanocomposite. Colloid Surface A 322(1-3): 47-53.
- Chung K T (2016) Azo dyes and human health: A review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 34(4): 233-261.
- Nakashima K, Kubota F, Maruyama T, Goto M (2005) Feasibility of ionic liquids as alternative separation media for industrial solvent extraction processes. Ind Eng Chem Res 44(12): 4368-4372.
- Parikh J, Rathore J, Bhatt D, Desai M J (2013) Clouding behavior and thermodynamic study of nonionic surfactants in presence of additives. J Disper Sci Technol 34(10): 1392-1398.
- Ojeda B, Rojas S (2009) Separation and preconcentration by a cloud point extraction procedure for determination of metals: an overview. Anal Bioanal Chem 394: 759-782.
- Kjellin URM, Claesson PM, Linse P (2002) Surface properties of tetra (ethylene oxide) dodecyl amide compared with poly(ethylene oxide) surfactants. 1. effect of the headgroup on adsorption. Langmuir 18: 6745-6753.
- Bezerra MA, ArrudaMAZ, Ferreira SLC (2005) Cloud point extraction as a procedure of separation and pre‐concentration for metal determination using spectroanalytical techniques: a review. Appl Spectrosc Rev 40(4): 269-299.
- Villemin D, Didi MA (2013) Extraction of rare earth and heavy metals, using ionic solvents as extraction medium (A Review). Orient J Chem 29(4): 1264-1284.
- Mehdi H, Binnemans K, Hecke K, Meervelt L, Nockemann P (2010) Hydrophobic ionic liquids with strongly coordinating anions. Chem Commun 46(2): 234-236.
- Taoxiang S, Gao S, Chen Q, Sheen X (2014) Investigation on the interactions between hydrophobic anions of ionic liquids and Triton X-114 micelles in aqueous solutions. Colloid Surface A 456: 18-25.
- Zhang S, Gao Y, Dong B, Zheng L (2010) Interaction between the added long-chain ionic liquid 1-dodecyl-3-methylimidazolium tetrafluoroborate and Triton X-100 in aqueous solutions. Colloid Surface A 372(3): 182-189.
- Thakkar K, Patel V, Ray D, Pal H, Aswal VK, et al. (2016) Interaction of imidazolium based ionic liquids with Triton X-100 micelles: investigating the role of the counter ion and chain length. RSC Adv 43: 36314-36326.
- Kodama K, Tsuda R, Niitsuma K, Tamura T, Ueki T, et al. (2011) Structural effects of polyethers and ionic liquids in their binary mixtures on lower critical solution temperature liquid-liquid phase separation. Polym J 43: 242-248.
- Selwent A, Łuczak J (2016) Micellar aggregation of Triton X-100 surfactant in imidazolium ionic liquids. J Mol Liq 221: 557-566.
- Talbi Z, Haddou B (2014) Cationic dye removal from aqueous solutions using ionic liquid and nonionic surfactant-ionic liquid systems: a comparative study based upon experimental design. Chem Eng Comm 201(1): 41-52.
- Dharaiya N, Parmar A, Bahadur P (2015) An efficient cloud point extraction method for the separation of congo red using Triton X-100 in the presence additives. Indian J Chem 54(5): 627-637.
- Didi MA, Sekkal AR, Villemin D (2011) Cloud-point extraction of bismuth (III) with nonionic surfactants in aqueous solutions. Colloid Surface A 375: 169-177.
- Chawla J, Mahajan RKJ (2011) Cloud point studies of tween and glycol in the presence of salts. Disper Sci Technol 32(6): 822-827.
- Kadam Y J (2010) Clouding and hydrodynamic behavior of pluronic-surfactant interaction in aqueous salt solutions. Disper Sci Technol 31(7): 870-876.
- Haddou B, Canselier JP, Gourdon C (2006) Cloud point extraction of phenol and benzyl alcohol from aqueous stream. Sep Purif Technol 50(1): 114-121.
- Rub MA, Youbi AO (2015) Influence of additives (inorganic/organic) on the clouding behavior of amphiphilic drug solutions: Some thermodynamic studies. J Saudi Chem Soc 19(3): 292-300.
- Materna K, Szymanowski J (2002) Separation of phenols from aqueous micellar solutions by cloud point extraction. J Colloid Interface Sci 255(1): 195-201.
- Sado G, Christine Sado M (2000) Les plans d’expériences de l’expérimentation à l’assurance qualité, Nouvelle édition, AFNOR.
- Goupy J (2001) Introduction aux Plans d'expériences, Dunod, Paris, 303.
- Bhatt DR, Maheria KC, Parikh J K (2016) Determination of thermodynamics and design parameters for ionic liquid-induced cloud point extraction of Coralene red dye. Int J Environ Sci Technol 13: 589-598.
- Mousavi R, Nekouei F (2011) E J Chem 8(4): 1606-1613.
- Haddou B, Bouberka Z, Derriche Z, Taibi A, Bouabdesselam H (2007) Separation of Neutral Red and Methylene Blue from Wastewater using Two Aqueous Phase Extraction Methods. Sep Sci Technol 42(12): 2677-2691.
- Mondal S, Purkait MK, De S (2018) Advances in dye removal technologies. Green Chemistry and Sustainable technology Springer Singapore: 323-350.
- Gurkan R, Yılmaz O (2013) Cloud point extraction of trace cyanide from environmental waters for indirect flame atomic absorption spectrometric determination. Toxicol Environ Chem 95(9): 1455-1469.
- Heydari R, Hosseini M, Zarabi S (2015) A simple method for determination of carmine in food samples based on cloud point extraction and spectrophotometric detection. Spectrochim Acta A 150: 786-791.
© 2020 Didi MA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






