- Submissions

Full Text
Academic Journal of Engineering Studies
Normal Electrochemical Deposition NiFe: Experiments And Theory
Tikhonov Robert Dmitrievich*
Department of Micro-Electronics, Russia
*Corresponding author: Tikhonov Robert Dmitrievich, Department of Micro-Electronics, Russia
Submission: July 09, 2020; Published: July 20, 2020
.jpg)
ISSN:2694-4421 Volume1 Issue3
Abstract
Due to heating of the electrolyte is an excluded abnormal co-deposition alloy components and reduced variation of process parameters to achieve optimal magnetic properties Ni81Fe19 films of magnetic field concentrators. Proposed chloride electrolyte pH adjusted with hydrochloric acid, which provides congruent electrochemical deposition of perm alloy at heating and stirring. Magnetic properties of perm alloy films are very sensitive to the variation of component relationships of 4.26. Control of accuracy of preparation of chloride electrolyte for electrochemical deposition of Ni, Fe conducted using spectrophotometry. It is shown that the selection process of cooking the electrolyte for electrodeposition of Ni81Fe19 alloy and temperature allow to get normal, congruent electrochemical deposition of perm alloy films. It has been established that the anomalous character of perm alloy deposition associated with the main feature of iron ions-the existence of variable Valence iron with two or three values in the charge of ions during the hydrolysis of iron salts.
Keywords: Perm alloy; Magnetic field concentrators; Electrochemical deposition; Chloride electrolyte; Spectrophotometric monitoring
Introduction
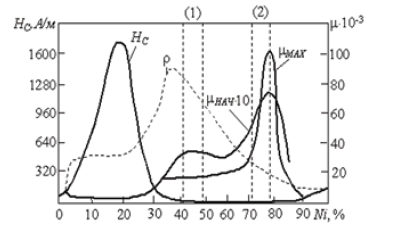
Ni, Fe alloy has high maximum relative magnetic permeability μ~100 000, low coercivity 1 Oe (Figure 1). These properties are manifested in a narrow range of changes in the composition 80% Ni [1]. The possibility of strengthening the magnetic induction to five orders of magnitude is of great interest to developers magneto sensitivity integrated microsystems.
Figure 1: Magnetic properties of permalloy [1].
Abnormal deposition of permalloy
A nickel-iron alloy was obtained in [2] by the electrodeposition of chloride electrolyte with a total concentration of iron and nickel (Fe + Ni) =1mol/l and CFe/C(Fe + Ni). Anomalous deposition was performed for the first time at a lower deposition rate for more noble nickel compared to iron, which was explained based on the influence of iron on the deposition of nickel. The degree of anomalous precipitation depends on temperature, pH, and the use of pH buffers [3]. Simple sulfate or chloride electrolytes in a perm alloy bath must be used in the pH range from 3 to 5. At values below 3, the current efficiency falls to invalid values, and above 5, Fe3+ precipitates form and drop out into the sediment [4] shows that in the mechanism of anomalous co-deposition of Ni and Fe, a high content of Fe in the alloy is connected with the formation of Fe(OH)2 and hydroxide precipitation due to a localized increase in pH at the surface when hydrogen is selected. Anomalous co-deposition occurs due to the suppression of nickel (Ni2+) discharge, which occurs when the surface pH is sufficiently high to form iron hydroxide. The adsorption of iron hydroxide suppresses the deposition of nickel but allows the discharge of iron (Fe2+). A mathematical model for anomalous alloy deposition onto a rotating disk electrode has been developed [5]. The role of metal‐hydroxide ions in the deposition of single metals, reported by other researchers, is extended to co-deposition.
Two‐step reaction mechanisms involving adsorbed monovalent intermediate ions for the electrodeposition of iron and nickel as single metals can be combined to form a predictive model for the co-deposition of iron‐nickel alloys [6]. Inhibition of the more noble nickel in the presence of iron is caused by preferential surface coverage of the adsorbed iron intermediate resulting from a difference between the two elements in Tafel constant for the electro sorption step. The analysis shows that changes in surface pH with potential are not necessary for iron‐rich (anomalous) deposits, but that variations in pH from one electrolyte to another may influence deposit composition.
The electrodeposition of Fe-Ni alloys was performed Galvano statically in the sulfate solutions of pH 1-3 at 40 °C and the alloy deposition behavior was compared with that of Zn-iron-group metal alloys to investigate their co-deposition mechanism [7]. The anomalous co-deposition behavior in Fe-Ni alloy deposition was evidently dependent on the pH buffer capacity of the solutions. This can be explained in terms of the preferential adsorption of Fe OH on the deposition sites of more noble Ni due to the extremely smaller dissociation constant of Fe OH+ than NiOH+ in the multi-step reduction process of hydrated iron-group metal ions. Therefore, it is important to make clear the mechanism of anomalous deposition of Fe-Ni alloys. The hydroxide suppression model [4] and the mathematical model [5] have been proposed so far to explain the anomalous co-deposition of Fe with Ni. The hydroxide suppression model explained that noble Ni deposition was strongly suppressed in the presence of Fe(OH)2 preferentially formed and adsorbed on the cathode, while the mathematical model was based on the great difference in the dissociation constant between FeOH+ and NiOH+. However, no mechanism explaining completely for the anomalous co-deposition has been reported.
The growth rate of permalloy films versus content iron and nickel of chloride electrolyte
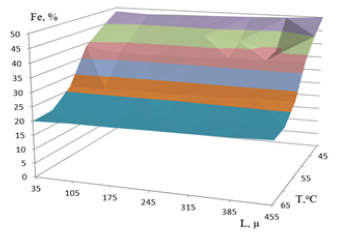
Effect of temperature of the electrolyte composition on the permalloy films: The combination of many factors influencing magnetic properties of permalloy, defines a wide range of their change and the difficulty of playing high magnetic parameters [8]. An empirical study of the process of electrochemical deposition permalloy allowed to obtain of magnetic field concentrators by the method of electrochemical deposition permalloy in local area using chip photoresist masks metallized surface of the silicon wafer [9]. Heat the electrolyte improves wettability, aligns the thickness 20µ of the hub by improving adhesion and reduces tension in the layer. Figure 2 presents the iron content dependence in permalloy film electrolyte temperature without mixing. The composition of the beleaguered film is determined by the temperature of the electrolyte. At a temperature of 40 °C composition of the films Ni51Fe49 and at temperatures of 60 and 65, respectively, Ni79,1Fe20,9, and Ni79,4Fe20,6. The temperature dependence of the composition is stabilized at 60 °C. The film is very neat and clean, with almost uniform composition on the surface of the hubs.
Figure 2: Iron content dependence of Fe in film of permalloy with electrolyte temperature ToC with distribution across the width L region.
The growth rate of permalloy films versus content iron and nickel of chloride electrolyte
According to Faraday's first law m=c I t, where m is the mass of metal filtration on electrodes, g; p-electrochemical equivalent, g/(Ah); I-current, A; t-time of the process, h. The current and time defined in the process and electrochemical equivalent depends on the nature of the metal and solvent, electrolyte temperature, metal ion activity. The actual amount of reduction at the cathode metal output is defined for the current. To increase the output of cathode electrolyte compositions are developed with various additives. In chloride electrolyte corresponding to the content of iron and nickel alloy Ni81Fe19 increase cathode output achieved additives main salts [10], that increases the concentration of nickel and iron atoms without changing their relationship up to the limit solubility of chlorides in the water.
Figure 3 presents the speed dependency of electrochemical deposition of permalloy films current density 6-26mA/cm2 at temperature chloride electrolyte 70 °С, for three electrolytes with the relationship of the content of nickel and iron atoms 4.26 and Mole fraction iron content 29; 58; 87mmol/l. Bar-dotted line 1 corresponds to Faraday's law when calculating for the alloy Ni81Fe19. Cross marked point 2 is congruent Ni42Fe58 alloy deposition. Figure 3 shows that increasing concentrations of nickel and iron in the electrolyte with Fe/Ni=19/81 and Fe=0.087 mol/l growth rate increases and not film goes in saturation when approaching a film composition congruent composition of the electrolyte. Space allocated congruence lines. Film growth rate approaching the limit, the corresponding law of Faraday, i.e. the cathode output approaches unity.
Figure 3: Dependence of speed electrochemical deposition of permalloy films versus current density in chloride electrolyte.
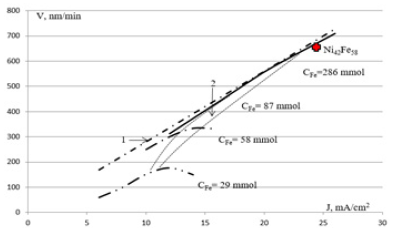
When this film is firmly linked to the sublayer of nickel, and mechanical stresses in thick films lead to warping silicon wafer. To reduce mechanical stress process of electrochemical deposition alloy NILO Ni42Fe58, has the same coefficient of thermal expansion with silicon and installed a cross point congruent NILO alloy deposition. Within the range of current density changes from 16 up to 24mA/cm2 growth speed of the film grows from 400 to 700nm/min, and the almost constant and corresponds to the composition of the electrolyte with a ratio of concentrations of iron and nickel 19/81. In this multidimensional space of parameters of electrochemical deposition can be called congruent, unlike the anomalous co-deposition.
Electrochemical deposition of Ni, Fe alloy at a temperature of 70 °C
A very interesting composition dependence on the temperature was obtained for the electrolyte for the following composition, g/l: FeCl2 4H2O-11; NiCl2 6H2O-56; H3BO3-25; C7H5NaNO3S-1.5; and HCl-2.7ml/l.
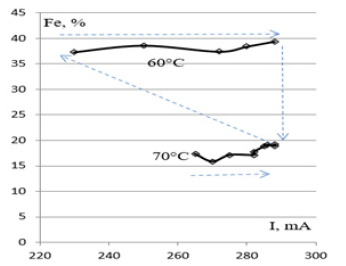
Holding 12 processes at the electrolyte temperature of 60°C and 70°С strongly modifies the iron content. Figure 4 shows that the sequence of processes and current does not affect the composition, which is determined only by the temperature. The permalloy films deposited at the electrolyte temperature of 60 °С contain 39.4% iron. The permalloy films deposited at the electrolyte temperature of 70 °С contain 19.2% iron. Based on the results of the X-ray spectral analysis for the deposition of the same electrolyte, all the values of current showed twice higher iron content at 60 °С. The film deposition speed of different compositions depending on the current is shows a slight dependence on the current magnitude, after growing simultaneously for samples obtained at the temperatures of 60 and 70 °С. The electrochemical equivalent of permalloy deposition for the electrolyte has a single value for the films based on the growth rate. However, doubling of the iron content in the film at a lower temperature may be associated with the charge value of the iron ion. Voltage drop between electrolytic cathode and anode at a temperature of 70 °С compared to 60 °С decreases, which indicates that the greater the conductivity of the electrolyte through the charge of ions.
Figure 4: Iron content dependence in permalloy films on the current in chloride electrolyte with CNi/CFe=4.26 at temperatures of 60 °C and 70 °С, pH=1.9.
This concept is confirmed by the pH dependence on the FeCl2 4H2O from concentration. pH is decreased by dissolving FeCl2, which means that because of the dissociation of salts in water, ions and hydroxyls are generated. A complete dissociation of the formed Fe(OH)2 and 2HCl is obtained without altering the pH. Upon single ionization of FeCl2 as (Fe2+Cl-)+ for the second chlorine ion, in collaboration with the water, Cl-+H2O forms HCl and excess OH-. According to the Le Chatelier principle, reduction of the concentration of ions should lead to further disassociation of water molecules into ions. If a molecule participates in the formation of ions from the electrolyte, then another ion accumulates in the solution and the pH changes.
Electrochemical equivalents for metals with double valence give a natural change in the deposition rate relative to their valence. When the current is determined by the electrochemical charge ions, iron, nickel and cobalt are precipitated 1.5 times slower than in the bivalent case.
With this ratio of electrolyte components, the concept of anomalous deposition changes from suppression of the deposition of nickel iron hydroxide to doubling of the deposition rate for iron ions (FeCl)+ compared to the doubly charged Fe2+. At the temperature of 70 °С, a concentration ratio change occurs at the expense of full ionization of singly charged ions (FeCl)+, and the deposition of the electrolyte with doubly charged Fe2+ is observed, corresponding to the normal, conventional notion of the deposition of nickel-iron alloy. A new phenomenon of full ionization of ferric chloride in the electrolyte is observed at the temperature of 70 °C.
The reviewed explanation of the anomalous deposition of nickel-iron alloy based on the hydrolysis of ferric chloride not only explains this phenomenon, which was discovered 60 years ago, but also provides the solution to the problem of obtaining films under the common reaction of normal structure deposition. At the temperature of 70 °С, the composition of the chloride electrolyte with a concentration ratio of CNi/CFe=4.26 led to the obtainment of electrochemical permalloy Ni81Fe19 films. Numerous previous studies with a wide range of electrolyte composition had different relative contents of ions (FeCl)+ and Fe2+ that led to a large variation in the entire film.
Figure 5: Electrochemical deposition of NiFe alloy films containing 19% iron from electrolytes with concentrations Fe of 1. 0.0037 mol/l at a temperature point, 2. 0.054 mol/l at a temperature of 65 °C, 3. 0.11 mol/l at a temperature of 70 °С, and 4. 0.22 mol/l at a temperature of 60 °C.

Figure 6: Electrochemical deposition of NiFe from chloride electrolyte.

Electrochemical deposition of NiFe alloy films of chloride electrolyte containing low concentration of salts
Performing the NiFe alloy electrochemical deposition using iron ions with changing electrolyte temperature allows for a new approach to the fabrication of the electrolyte. The charge of the ions in the electrolyte may depend not only on the temperature but also on the iron concentration. It is well-known that reduction of the concentration leads to strengthening of the electrolytic dissociation of salts. For the content of iron in permalloy films obtained [11] by diluting chloride electrolyte, it should be found that films with permalloy Fe19Ni81 are obtained by the use of simple electrochemical chloride electrolyte with CNi/CFe=4.26 and concentrations of Fe+2-0.004mol/l. Consequently, at these concentrations of nickel and iron in molten chloride, the nickel and iron ions with charges Ni2+, and Fe2+ have the same amounts in the cathode discharge.
Obtaining weak solutions of permalloy films not only increases the induced electrolytic salts and ion content but also leads to doubly charged ions instead of singly charged ions. Electrochemical deposition of permalloy films from chloride electrolyte with СNi/СFe= 4.26 and concentrations of Fe+2-0.004mol/l at room temperature allows one to obtain films with congruent electrolyte composition. The use of dilute chloride electrolyte for NiFe deposition with a ratio of СNi/СFe= 4.26 confirms the principle that the charge of iron ions influences the composition of permalloy films. The effect of the temperature of the electrolyte on the ability to obtain permalloy films containing 19% iron is presented for selected concentrations of iron in solution in Figure 5. At low concentrations of nickel and iron chlorides, the room temperature deposition rate is significantly less than that at higher concentrations and 70 °С.
Normal electrochemical deposition of NiFe films
Electrochemical deposition of chloride electrolyte and content of nickel and iron atoms in relation to 4.26 corresponding alloy Ni81Fe19 at a temperature of 70 °С gave the composition dependence of permalloy films in the current range 270-410mA, shown in Figure 6. Changing the current process does not lead to a noticeable change in the content of iron and nickel film. Current value is selected when changing the content of iron and nickel due to additives in electrolyte FeCl2 4H2O leads to modify the content of the film.
Chemical Processes in Сhloride Electrolyte
Hydrolysis of ferric chloride
The interaction of ions of salts with water leads to the formation of a weak electrolyte resulting from the hydrolysis of salt [12]. Salt ions bind with water-generated ions, either hydrogen ions H+ or OH-. According to the Le Chatelier principle, a decrease in the concentration of the ions should lead to further disintegration of water molecules into ions. If an ion participates in the formation of ions from the electrolyte, then another ion accumulates in the solution, and the pH changes. To strengthen salt hydrolysis, the solution is diluted and heated. According to the law of mass action, supplying one of the products of the hydrolysis to the solution decreases the hydrolysis of the salt. When a product of hydrolysis is removed, the hydrolysis of the salt is enhanced. The dissolution of ferric chloride in water [5] do hydrolysis of ferric (II) chloride occurs in acidic water in the presence of cations.
FeCl2 + H2O ↔ Fe(OH)Cl +HCl.
Is formed principal salt FeOHCl.
FeCl3 + H2O ↔ Fe(OH)Cl2 +HCl.
Is formed principal salt FeOHCl2 Like all other compounds of divalent iron, iron (II) hydroxide has restorative properties, and in the presence of O2 and H2O, the dissolved oxygen is dissolved to iron (III) hydroxide over time when standing in air:
4Fe(OH)2 + O2 + 2H2O=4Fe(OH)3
Iron (III) hydroxide, with the formula Fe2O3 nH2O, has a reddish-brown color and is not soluble in water.
The interaction of a brown sludge of iron (III) hydroxide with a solution of hydrochloric acid leads to the dissolution of the precipitate and the formation of a yellow solution of iron (III) chloride.
Fe(OH)3 + 3HCl=FeCl3 + 3H2O
Hydrochloric acid in electrolyte additive excludes formation of precipitate Fe(OH)3. The electrolyte is illumined and stabilizes. Nickel chloride hydrolyzed completely and forms ions with two сharges.
Ionic balance in the hydrolysis of FeCl2
Seung ML [13] discusses the ionic equilibrium in the FeCl2 electrolyte through thermodynamic calculations of equilibrium constants, considering the adverse reactions, as well as mass and charge balance equations.
The solution of FeCl2 4mol/l at room temperature mostly contains singly charged ions (Fe2+Cl-)+, a smaller content of Fe2+ ions, a small amount of neutral molecules and a very slight amount of hydroxide ions (Fe2+OH-)+. Addition of hydrochloric acid increases the content of the singly charged ions (Fe2+Cl-)+ and FeCl2° neutral molecules and reduces the amount of doubly charged Fe2+ ions.
When used for the deposition of permalloy, the FeCl2 concentration on the order of 0.1mol/l solution contains equal concentrations of singly charged ions (Fe2+Cl-)+ and fewer Fe2+ ions, with the difference in the concentration of these ions increasing upon the addition of hydrochloric acid. The contents of the neutral molecules FeCl2° and hydroxide ions (Fe2+OH-)+ are considerably smaller than those of the (Fe2+Cl-)+ and Fe2+ ions. For these calculations, the hydroxide and (Fe2+OH-)+ deposition should not have a significant impact. When the electrolyte is heated, increasing ionization is observed because the number of intermediate ions (Fe2+Cl-)+ can be smaller than the number of Fe2+ ions. The content of neutral molecules becomes very small.
The exception is the influence of three valency iron on electrodeposition of permalloy
Effect of ferric ion on the permalloy deposition process shall be considered an act of ferric ion in source electrolyte. Elimination of ferric iron precipitates using boric acid and filtration and suppresses education trivalent iron ions Fe3+ hydrochloric acid electrolyte ensure stability, eliminate anomalous deposition process and congruent is a characteristic feature of co-deposition of NiFe alloy in which the mol ’nymi composition of the precipitate corresponds to composition of the electrolyte.
Model Discharge of Iron and Nickel Ions on the Cathode
Experimental results obtaining permalloy films specified composition [10,11] find scientific justification as an exception at the cathode deposition from chloride electrolyte once charge ions of bivalent and trivalent iron. Have not been considered in the literature component ratio of job in the electrolyte is equal to a specified composition component in the film. Has not been the stabilization of the electrolyte. None of the researchers linked the anomalous deposition of iron ions with charge. Therefore, the deposition of the permalloy films was not repeatable process. Electrochemical deposition of NiFe depends on the charge of ions of Fe in the electrolyte. A charge ion depends not only on temperature but also on the concentration of Fe. Decreased concentration is well known to exacerbate electrolytic salt dissociation. To ascertain the nature of the events taking place in chloride electrolyte, we studied and deposition of thin films at room temperature (Figure 7).
Figure 7: The dependence the composition of the NiFe film from the electrolyte composition.
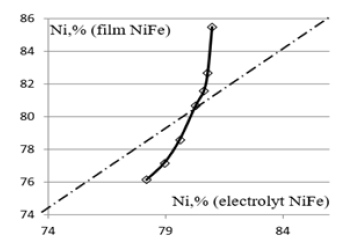
At the probability current density, the discharge of ions depends on their concentration, and contributions to electric current from their charge. When you change the current density occur a change the contribution of different ions and the change in the composition of the sediment. Discharge of ions at the cathode determines current cathode. Content of ions in the electrolyte sets the composition of sediment. One charge ion gives you more sediment than two charge ion. Changing the current changes, the ratio of the ions in the sludge. This determines the dependence of the whole sediment from current. In the same spirit nickel and iron ions occurs congruent permalloy deposition. The composition of the film does not depend on current.
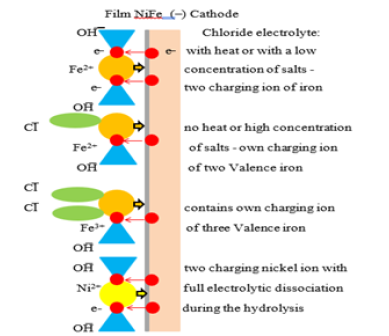
Conditional discharge mechanism of nickel and iron ions at the cathode is shown in Figure 8, with an indication of the charge of ions and the characteristics of chloride electrolyte with heat, cleaning or with low salt concentrations of ferric chloride.
Figure 8: The discharge of ions of iron and nickel on the cathode at electrochemical deposition of permalloy
The Magnetic Properties of Permalloy Films
The magnetic properties of permalloy films prepared by the local electrochemical deposition of a chloride electrolyte, with a specified ratio of Ni/Fe=4.26, were investigated. Magnetic properties of the films permalloy described in article [14]. For the sample magnetic field concentrators on silicon wafers, which is shown in Figure 6, specific magnetic magnetization and coercivity provided depending on the concentration of iron in permalloy films in Figure 9. The effect of the Fe content films on the magnetization of permalloy was studied. Figure 9 shows that a small coercive force of less than 1Oe was obtained in the NiFe alloy when the Fe concentration ranged from 16% to 24%.
Figure 9: The dependence of the magnetization of magnetic density (Bs/h) and coercive force (Hc) on the Fe content in the film of the NiFe permalloy when the Tikhonov method is used for local electrochemical deposition.
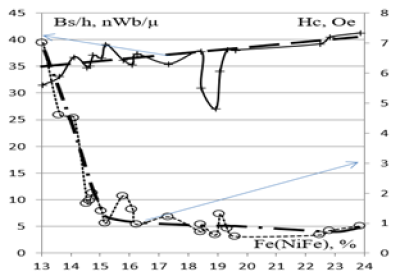
Meanwhile, the specific magnetization of the films was increased slightly from 35 to 41nWb/µ. When the Fe concentration ranged 19% the specific magnetization of the films decreased 27nWb/µ. When the concentration of iron 13% coercivity increases to 7Oe.Permalloy films used to fabricate magnetic field concentrators improved the sensitivity of the magnetic semiconductor microsystems. The magnetization of multielement concentrators extended the range of the magnetic field before achieving saturation.
Conclusion
Summing up the results of the analysis of chemical characteristics of hydrolysis of ferric chloride, it should be noted that researchers in over 65 years [2] conducted NiFe alloy plating and receive preferential deposition of iron in relation to nickel in all formulations electrolyte. Electrolytes used [15-29] is characterized by a wide range of molar relationship of nickel and iron. The cause of the anomalous co-deposition of preferential deposition of iron sources previously not associated with the main feature of iron ions-the existence of variable Valence iron one and two values in the charge of ions during the hydrolysis of iron salts [30].
Selection of chloride electrolyte with attitude CNi/CFe=4.26, development of technology of preparation of electrolyte and determination of optimal electrolyte temperature revealed the mechanism of abnormal deposition because of incomplete ionization of atoms of iron and ensure that in the film the relationship CNi/CFe=4.26 at room temperature, without mechanical stress, with uniform structure and high magnetic parameters without high temperature annealing. The effect of charging of iron ions in the electrolyte is set to congruent permalloy films deposition.
References
- (2004) Publication Number SMC-031, Special Metals Corporation.
- Korovin NV (1957) О katodnom processe pri elektroosajdenii splava jelezo-nikel. Journal neorganic chimii 2(9): 2259-2263.
- Вrenner А (1963) Electrodeposition of alloys. Academic Press, New York.
- Dahms H, Croll IM (1965) The anomalous co-deposition of iron‐nickel alloys. J Electrochem Soc 112(8): 771-775.
- Hessami S, Tobias CW (1989) A mathematical model for anomalous co-deposition of nickel‐iron on a rotating disk electrode. J Electrochem Soc 136(12): 3611-3616.
- Matlosz M (1993) Competitive adsorption effects in the electrodeposition of iron‐nickel alloys. J Electrochem Soc 140(8): 2272-2279.
- Nakano H, Matsuno M, Oue S, Yano M, Kobayashi SH, et al. (2004) Mechanism of anomalous type electrodeposition of Fe-Ni alloys from sulfate solutions. Materials Transactions 45(11): 3130-3135.
- Tikhonov RD (2015) Electrodeposition of Ni-Fe alloys for the production of integrated circuits. Surface 23(4): 13-20.
- Tikhonov RD (2017) Normal electrochemical deposition of NiFe films. Advances in Research 11(2): 1-10.
- Robert Tikhonov (2019) Congruent electrochemical deposition of NiFe alloy. Lambert Academic Publishing p. 193.
- Tikhonov RD (2018) The effect of ion charge on ferric chloride hydrolysis during electrochemical deposition of NiFe alloy. British Open Journal of Chemical Sciences 2(1): 1-14.
- Korovin NV, Vyshay M (1998) Obtschay chimiy. p. 559.
- Seung ML (2006) Use of the bromley equation for the analysis of ionic equilibria in mixed ferric and ferrous chloride solutions at 25 °C. Metallurgical and Materials Transections B 37(2): 173-179.
- Tikhonov RD (2018) Magnetic properties of permalloy films deposited electrochemically by the Tikhonov method. British Open Journal of Chemical Sciences 2(2): 1-10.
- Li Z, Sun X, Zheng Y, Zhang H (2013) Microstructure and magnetic properties of micro NiFe alloy arrays for MEMS application. J Micromech Microeng 23(8):1-6.
- Moniruzzaman M, Shorowordi KM, Ashraful A, Taufique MFN (2014) Fe-Ni alloy elecrodeposition from simple and complex type sulfate electrolytes containing Ni/Fe ratio of 1 and 12. Journal of Mechanical Engineering 44(1): 50-56.
- Cao Y, Wei GY, Ge HL, Meng XF (2014) Study on preparation of NiFe films by galvanostatic electrodeposition. Surface Engineering 30(2): 97-101.
- Tabakovic I, Gong J, Riemer S, Kautzky M (2015) Influence of surface roughness and current efficiency on composition gradients of thin NiFe films obtained by electrodeposition. J Electrochem Soc 162(3): 102-108.
- Spada ER, Oliveira LS, Rocha AS, Pasa AA, Zangari G, et al. ((2004)) Thin films of FexNi1-x electroplated on silicon (100). Journal of Magnetism and Magnetic Materials pp. E891-E892.
- Giovanni Z (2015) Electrodeposition of alloys and compounds in the era of microelectronics and energy conversion technology. Materials Science and Engineering 5(2): 195-218.
- Dragos O, Chiriac H, Lupu N, Grigoras M, Tabacovic I (2016) Anomalous co-deposition of fcc NiFe nanowires with 5-55% Fe and their morphology, crystal structure and magnetic properties. J Electrochem Soc 163(3): 83-94.
- Deepthi KA, Balachandran R, Ong BH, Tan KB, Wong HY, et al. (2016) Physical and electrical characteristics of NiFe thin films using ultrasonic assisted pulse electrodeposition. Applied Surface Science 360: 519-524.
- Schiavone G, Murray J, Perry R, Mount A, Marc R (2017) Integration of electrodeposited Ni-Fe in MEMS with Low-temperature deposition and etch processes. Materials (Basel) 10(3): 323-331.
- Wang F, Li L, Qiu Sh, Wang H (2017) Ferronickel preparation using Ni-Fe co-deposition process. Materials, Metallurgy, Chemical and Environmental Engineering 23: 3072-3078.
- Torabinejad V, Aliofkhazraei M, Assareh S, Allahyarzadeh MH, Rouhaghdam S (2017) Electrodeposition of Ni-Fe alloys, composites, and nano coatings-A review. Journal of Alloys and Compounds 691: 841-859.
- Białostocka A, Klekotka U, Kalska SB (2018) Modulation of iron-nickel layers composition by an external magnetic field. Chemical Engineering Communications 206(6): 804-814.
- Kashiwa Yu, Nagano N, Takasu T, Kobayashi SH, Fukuda K, et al. (2018) Effects of electrolyte composition and additives on the formation of invar Fe-Ni alloys with low thermal expansion electrodeposited from sulfate bath. Tetsu To Hagane 104 (10).
- Zubar TI, Fedosyuk VM, Trukhanov AV, Kovaleva NN, Astapovich KA, et al. (2019) Control of growth mechanism of electrodeposited nanocrystalline NiFe films. J Electrochem Soc 166(6): 173-180.
- Cesiulis H, Tsytsaru N, Podlaha EJ, Li D, Sort J (2019) Electrodeposition of iron-group alloys into nanostructured oxide membranes: Synthetic challenges and properties. Current Nanoscience 15(1): 84-99.
- Yanai T, Eguchi K, Koda K, Kaji J, Aramaki H, et al. (2018) Investigation of coercivity for electroplated Fe-Ni thick films. AIP Advances 8(5): 056123.
© 2020 Tikhonov Robert Dmitrievich. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






