- Submissions

Full Text
Annals of Chemical Science Research
Resins as Scavengers to Control the Metal Impurities in Active Pharmaceutical Ingredients (APIs)
Uday Kumar Neelam, Narasimha Achary Darshanoju, Syamaiah Kaveri, Sai Krishna Yenneti and Mahender Rao Siripragada*
R&D Centre, Neuland Laboratories Ltd., India
*Corresponding author:Mahender Rao Siripragada, R&D Centre, Neuland Laboratories Ltd., Bonthapally Village, Telangana, 502313, India
Submission: June 20, 2023;Published: July 12, 2023

Volume4 Issue2July , 2023
Abstract
To overcome the unmet needs of patients, the production of active pharmaceutical ingredients (APIs) with limited lead timelines has become critical. Along with timelines to secure robust, economical and greener processes, the utilization of metal catalysts (palladium, rhodium and ruthenium catalysts) has become vital in the pharmaceutical industry. The presence of metal impurities in APIs can pose significant risks to human health and compromise the quality of pharmaceutical products. According to the ICH Q3D guideline, the permitted daily exposure (PDE) for metals in APIs is established based on toxicological data and the PDE values are expressed in micrograms per day and vary for different metals [1,2]. It is important to note that the acceptable limits for metal impurities can vary depending on factors such as the route of administration, patient population, and potential toxicity of the metal. In general, the metal impurities in APIs must be controlled to low levels (<10μg/g) [3]. Therefore, it is crucial to develop effective purification methods to reduce metal content in APIs. Resins have emerged as valuable tools in the pharmaceutical industry for the selective removal of metal impurities. This review provides an overview of the use of resins for the reduction of metals in APIs..
Mini Review
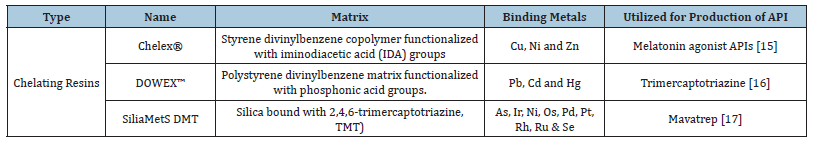
Metals, such as transition metals and heavy metals, can be present as impurities in APIs due to various reasons, including raw material contamination, catalyst residues from synthesis processes, or interaction with equipment during manufacturing. These metal impurities can be harmful and have potential toxicological effects on human health. The use of resins for the reduction of metals in active pharmaceutical ingredients (APIs) is an important aspect of pharmaceutical manufacturing to ensure the purity, quality, and safety of pharmaceutical products. By effectively removing metal impurities, resins play a crucial role in meeting regulatory requirements and ensuring patient well-being. Further research and development in resin technology and process optimization are necessary to advance metal reduction methods in pharmaceutical manufacturing. Number of cost-effective and scalable methods have been developed to remove the metal impurities [4-11]. Merck group and Astra Zeneca developed carbon or silica gel adsorbents to remove these metal impurities [12,13]. Astra Zeneca successfully demonstrated this application on polit scale [13]. Resins functionalized with chelating ligands, such as iminodiacetic acid (IDA) or iminodiacetate (IDA) resin, are commonly employed for metal reduction [14]. These resins possess high affinity for metal ions due to their specific coordination chemistry. Metal ions bind to the resin’s chelating groups, allowing for their selective removal from the API solution. The resin’s capacity, selectivity, and compatibility with the API solution are critical considerations during resin selection (Figure 1). Some of the examples of resins were provided in the below Table 1.
Figure 1:General mechanism for chelation of metal to scavenger.
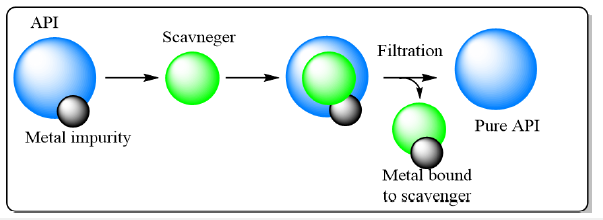
Table 1:Some examples of metal scavengers/ resins used in production of APIs.
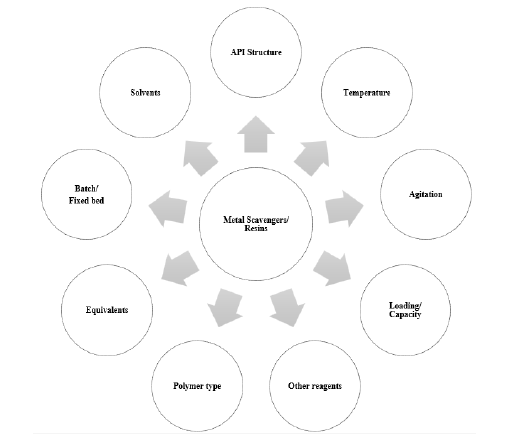
The process involves passing the API solution through a resinpacked column, where metal impurities interact with the resin while the desired API and other impurities pass through. The bound metal ions can be subsequently eluted from the resin using appropriate elution solutions. Resins for metal reduction are also often used in combination with other purification techniques, such as filtration, chromatography, or precipitation, to achieve the desired purity level for APIs. These multiple purification steps help ensure that the final product meets regulatory requirements and quality standards. The choice of resin and operating conditions (e.g., pH, flow rate) depends on the specific metal impurities and the API being processed. Optimization of resin selection and process conditions (equivalents of scavenger/resin, reaction temperature and pH of the aqueous reaction mixtures (Figure 2) [18,19] is essential to ensure efficient metal reduction. Factors such as resin capacity, selectivity and stability under process conditions should be evaluated. Additionally, the compatibility of the resin with the API and the specific metal impurities must be considered [18].
Figure 2:Factors involved in effective metal scavenging.
Manufacturers are responsible for conducting appropriate analytical testing to ensure compliance with the established metal impurity limits. These tests typically involve validated analytical methods, such as atomic absorption spectroscopy (AAS), [20] inductively coupled plasma (ICP) techniques, [21] or other suitable analytical techniques capable of accurately detecting and quantifying metal impurities in APIs.
Conclusion
In conclusion, the use of resins for metal reduction in active pharmaceutical ingredients is an essential purification step in pharmaceutical manufacturing. These resins provide an effective means to remove metal impurities, thereby ensuring the safety and quality of pharmaceutical products. The choice of resin and process conditions should be optimized based on the specific requirements of the API and the target metal impurities.
Acknowledgements
The authors thank management of R&D, Neuland laboratories Private Limited for encouraging this review article.
References
- http://www.ich.org/
- Guideline on the specification limits for residues of metals catalysts or metal reagents, EMEA/CHMP/SWP/4446/2000.
- Guidance for Industry (Drug Substance Chemistry, Manufacturing, and Controls Information).
- Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash-a low-cost adsorbent. Ind Eng Chem Res 42(25): 6619-6624.
- Urawa Y, Miyazawa M, Ozeki N, Ogura K (2003) A novel methodology for efficient removal of residual palladium from a product of the Suzuki-Miyaura coupling with polymer-supported ethylenediamine derivatives. Org Process Res Dev 7(2): 191-195.
- Galaffu N, Man SP, Wilkes RD, Wilson JRH (2007) Highly functionalised sulfur-based silica scavengers for the efficient removal of palladium species from active pharmaceutical ingredients. Org Process Res Dev 11(3): 406-413.
- Pink CJ, Wong HT, Ferreira FC, Livingston AG (2008) Organic solvent nanofiltration and adsorbents; a hybrid approach to achieve ultra-low palladium contamination of post coupling reaction products. Org Process Res Dev 12(4): 589-595.
- Bullock KM, Mitchell MB, Toczko JF (2008) Optimization and scale-up of a Suzuki-miyaura coupling reaction: development of an efficient palladium removal technique. Org Process Res Dev 12(5): 896-899.
- Girgis MJ, Kuczynski LE, Berberena SM, Boyd CA, Kubinski PL, et al. (2008) Removal of soluble palladium complexes from reaction mixtures by fixed-bed adsorption. Org Process Res Dev 12(6): 1209-1217.
- Recho J, Black RJG, North C, Ward JE, Wilkes RD (2014) Statistical DoE approach to the removal of palladium from active pharmaceutical ingredients (APIs) by functionalized silica adsorbents. Org Process Res Dev 18(5): 626-635.
- Samiey B, Cheng CH, Wu J (2014) Organic-Inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: A review. Materials 7(2): 673-726.
- Wang L, Green L, Li Z, Dunn JM, Bu X, et al. (2011) Screening binary systems of chelating agents combined with carbon or silica gel adsorbents: The development of a cost-effective method to remove palladium from pharmaceutical intermediates and APIs. Org Process Res Dev 15(6): 1371-1376.
- Reginato G, Sadler P, Wilkes RD (2011) Scaling up metal scavenging operations for pharmaceutical pilot plant manufactures. Org Process Res Dev 15(6): 1396-1405.
- Miyamoto H, Sakumoto C, Takekoshi E, Maeda Y, Hiramoto N, et al. (2015) Effective method to remove metal elements from pharmaceutical intermediates with poly-chelated resin scavenger. Org Process Res Dev 19(8): 1054-1061.
- Prasad JS, Vu T, Totleben MJ, Crispino AG, Kacsur DJ, et al. (2003) Development of Jacobsen asymmetric epoxidation and sharpless asymmetric dihydroxylation methods for the large-scale preparation of a chiral dihydro-benzofuran epoxide. Org Process Res Dev 7(6): 821-827.
- Rosso WV, Lust DV, Bernot PJ, Grosso JA, Modi SP, et al. (1997) Removal of palladium from organic reaction mixtures by trimercaptotriazine. Org Process Res Dev 1(4): 311-314.
- Wells KM, Mehrman SJ, Abdel-Magid AF, Ferraro C, Scott L, et al. (2015) Synthesis of Mavatrep: A Potent Antagonist of Transient Receptor Potential Vanilloid-1 (TRPV1). Org Process Res Dev 19(11): 1774-1783.
- https://www.biotage.com/documents/metal-scavenger-user-guide
- Phillips S, Holdsworth D, Kauppinen P, Namara CM (2016) Palladium Impurity Removal from Active Pharmaceutical Ingredient Process Streams. Johnson Matthey Technol Rev 60(4): 277-286.
- Aleluia ACM, Nascimento MS, Santos AMP, Santos WNL, Santos AF Júnior, et al. (2023) Analytical approach of elemental impurities in pharmaceutical products: A worldwide review. Spectrochimica Acta Part B: Atomic Spectroscopy 205: 106689.
- Mittal M, Kumar K, Anghore D, Rawal KR (2017) ICP-MS: Analytical Method for Identification and Detection of Elemental Impurities. Curr Drug Discov Technol 14(2): 106-120.
© 2023 Mahender Rao Siripragada. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






