- Submissions

Full Text
Annals of Chemical Science Research
Thermal Expansion Behavior of the Alpo4- 5 Molecular Sieve – High Temperature XRD Studies
Manjusha J Gavhane1, Pallavi D Bhange2 and Bhange DS1*
1Department of Chemistry, Shivaji University, India
2School of Sciences, Sanjay Ghodawat University, India
*Corresponding author: Bhange DS, Department of Chemistry, Shivaji University, India
Submission: October 26, 2021;Published: December 16, 2021

Volume3 Issue1October, 2021
Abstract
High temperature X-ray diffraction studies have been carried out on calcined AlPO4-5 molecular sieve in the temperature range 298-673K in order to study the thermal expansion behavior of this material. Rietveld refinement analysis of AlPO4-5 structure against the powder X-ray diffraction data collected at different temperature were carried out to derive the unit cell dimensions. Unit cell volume increases upto 373K and then remains nearly constant upto 523K and then decreases marginally. The material exhibits a complex thermal behavior viz, it expands initially upto 523 K and then contracts. The strength of expansion is greater than the strength of contraction.
Keywords: Microporous materials; X-ray diffraction; Thermal expansion
Introduction
Generally, materials expand on heating and shows positive thermal expansion coefficients. The materials, which show negative coefficients of thermal expansion (NTE) upon heating, are reported in the literature [1]. This phenomenon arises due to the secondary structural or dynamic mechanisms taking place during heating that makes the normal thermal expansivity of chemical bonds ineffective. This phenomenon is due to the rotation of the polyhedra due to the thermal expansion of certain bonds in one or two dimensions in some cases and is also due to the transverse thermal motion of two-coordinated cation/anion. Compounds having two-coordinated cations in their structure are very rare. Cu2O is one of the examples of this type, which shows NTE below room temperature (RT) and positive thermal expansion above RT. The other oxidic materials, which show NTE, are microporous materials encompassing silicate zeolites and aluminophosphates. Zeolitic and Aluminophosphate (AlPO4) material show negative thermal expansion at high temperature due to the transverse thermal motion of the two-coordinated oxygen atoms and this dynamic rocking of essentially rigid polyhedra may be responsible for their thermal behavior [2].
The limited work has been conducted on the thermal expansion behavior of microporous alumino-phosphate materials as compared to their structural studies [3,4]. As these materials have been used as catalysts in various chemical and petrochemical industries, it is necessary to study the thermal expansion behavior of these materials within their operational temperature range (298-673K). We recently reported the thermal expansion behavior of silica polymorph of Mobil Five (MFI) molecular sieve and effect of heteroatom substitution on its thermal expansion behavior [5-8]. In the present work, we have studied the thermal behavior of AlPO4-5 molecular sieve (Figure 1) as a function of temperature using high temperature X-ray diffraction (HTXRD) technique in the temperature range 298-673K. Rietveld refinement of the X-ray powder patterns was carried out to extract the values of unit cell parameters, which were used to calculate the thermal expansion coefficients.
Figure 2: Structural view of AlPO4-5 along the ‘c’ direction.
Experimental
Synthesis of the AlPO4-5 molecular sieve under study was carried out using the procedure described elsewhere [9]. Obtained powder was calcined at 600 ˚C in air to remove template inside the pores. The phase purity of the prepared sample was checked using powder X-ray diffraction technique. The experimental set up and the optics used for HTXRD data collection were identical as reported elsewhere [8]. A heating rate of 10K min-1 and a soak time of 10min were applied. Powder X-ray diffraction patterns were collected in the temperature range 298-673K on the Philips X’Pert Pro 3040/60 diffractometer equipped with Anton Parr HTK 1600 attachment under static air environment. A small amount of sample was mounted on a platinum strip, which serves as the sample stage as well as the heating element. A Pt/Rh -13% thermocouple spot-welded to the bottom of the stage was used for measuring the temperature. Data was collected in the 2θ region 5-60˚ in the continuous mode with a step size of 0.0167 and a time 20 s/step using Ni filtered Cu Kα radiation (λ=1.5406Å) and X’celerator as detector. Diffraction patterns were collected at every 50K interval from 323 to 673K. Bragg-Brentano geometry was employed. α-Al2O3 standard (NIST, Gaithersburg, USA) was used for the calibration of the high temperature stage. The actual sample temperature was confirmed by comparing thermal expansion coefficients of Pt with its known values. Rietveld refinement [10] analysis of AlPO4- 5 structures against the powder X-ray diffraction data collected at different temperature were carried out to derive the structural changes as function of temperature. The GSAS [11] package and the EXPGUI graphical interface [12] were used for Rietveld refinement, which allows proper treatment of the instrumental aberration parameters, such as the goniometer shift and the sample displacement parameters. The starting atomic coordinates for the AlPO4-5 in the hexagonal symmetry (space group P6cc (No. 184)) were taken from literature [13]. The pseudo-Voigt peak profile function was chosen, and the peaks were truncated at 0.01% of the peak maxima. Background was refined with a Chebyshev polynomial function. An overall scale factor, the cell parameters, and the sample displacement parameter were simultaneously refined. While the thermal expansion coefficient along the three crystallographic directions a, b and c were calculated for all the scans using the formulae described in literature [8].
Results and Discussion
Powder X-ray diffraction pattern (not shown here) of calcined AlPO4-5 confirmed the sample to be single-phase material without any impurity phases. Figure 2 shows the multiple plots of HTXRD patterns of AlPO4-5 in the temperature range 298-673K at intervals of 50K from 323-673K. Peaks appearing at 2θ=39.75˚ and 46.35˚ are the (111) and (002) reflection of Pt sample holder respectively. The unit cell parameters a, c and the unit cell volume V of AlPO4-5 at various temperatures are shown in Figure 3,4 respectively. The error bars shown are according to estimated standard deviation (esd) from the Rietveld refinement, which corresponds to 95% confidence level. The thermal expansion coefficients calculated for different temperature ranges are given in Table 1. The values in Table 1 clearly show that there is negative thermal expansion along the ‘c’ axis within temperature range 298-323K. Although the material exhibits a very strong positive thermal expansion along ‘a’ axis (aa=27.51x10-6K-1) and a negative thermal expansion along ‘c’ direction (ac=-7.36x10-6K-1), yet the overall volume thermal expansion is positive and very strong (aV = 48.02x10-6K-1) in the temperature range 298-523K.
Figure 2: Multiple plot of XRD patterns as a function of temperature.

Figure 3: Variation of unit cell parameters ‘a’ and ‘c’ as a function of temperature.

Figure 4: Variation of unit cell parameters ‘a’ and ‘c’ as a function of temperature.
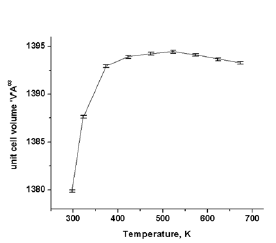
Table 1: Thermal expansion coefficients of AlPO4-5 molecular sieve in different temperature range.
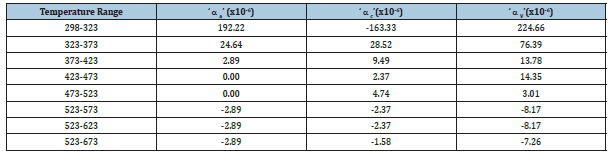
This initial expansion can be explained on the basis of the unfolding of polyhedrons comprising the structure, which may attain the saturation at 523K and after that it may contract due to the transverse thermal vibration of the bridging oxygen atoms in the Al-O-P bonds. In the temperature range 523-673K, the material exhibits overall negative thermal expansion. The thermal expansion coefficients along ‘a’ and ‘c’ axis are aa= -2.89x10-6K-1, ac=-1.58x10- 6K-1 respectively, and the volume thermal expansion coefficient is aV= -7.26x10-6K-1 (Table 1). The negative thermal expansion exhibited by this material is due to the transverse thermal vibration of the bridging oxygen atom. The contraction of the calcined AlPO4-5 material may originate from the availability of the empty cavities in the framework structure. With the aid of lattice dynamic calculations Tschaufesser and Parker [14] have predicted a correlation between negative thermal expansion and the nature of the channel systems. All molecular structures with a highly porous framework and two- or three-dimensional channel system should show negative thermal expansion according to Giddy et al. [15], which seems to result from the structural expansion in the space available in the pores and channels, on heating. These theoretical predictions, however, fail to explain the initial positive thermal expansion observed in our study. This study revealed a sharp negative thermal expansion of a c-axis in the studied temperature range. These results are similar to that observed by Park et al. [3] except some differences in magnitudes and the temperature range.
Conclusion
HTXRD studies on AlPO4-5 molecular sieve reveals a complex thermal behavior in the temperature range 298-673K studied. In the temperature range 298-523K, the lattice thermal expansion is very strong and positive (aV=48.02x10-6K-1) and in the range 523-673K, it is negative (-7.26x10-6K-1). These negative thermal expansion properties may find application in making electronic materials having zero thermal expansion.
Acknowledgement
Authors are thankful to University Grant Commission (UGC) New Delhi for the financial support [F.No.41-246/2012(SR)].
>References
- Ugbogu OC, Onyeagba RA, Chigbu OA (2006) Lauric acid content and inhibitory effect of palm kernel oil on two bacterial isolates and Candida albicans. African Journal of Biotechnology 5: 1045-1047.
- Imo C, Sunday OD (2020) Comparative Effects of Palm Kernel Oil, Olive Oil, Crude Oil and Honey on Lipid Profile, Body Weight and Hearts of Male Albino Rats. European Journal of Biomedical and Pharmaceutical Sciences 7(5): 84-90.
- Bredeson DK (1983) Mechanical Oil Extraction. Journal of the American Oil Chemists' Society 60(2): 211-213.
- Imo C, Arowora KA, Abu MS, Angbas FA (2020) Comparative Effects of Palm Kernel Oil, Olive Oil, Crude Oil and Honey on Liver Function of Male Albino Rats. European Journal of Pharmaceutical and Medical Research 7(5): 26-31.
- Sutapa M, Analava M (2009) Health effects of Palm Oil. Journal of Humidity and Ecology 26(3): 197–203.
- Imo C, Yakubu OE, Imo NG, Udegbunam IS, Onukwugha OJ (2018) Chemical composition of Xylopia aethiopica American Journal of Physiology, Biochemistry and Pharmacology 7: 48–53.
- Chemical Entities of Biological Interest a (ChEBI a).
- Koenig G, Lohmar E, Rupprich N (2005) Chloroacetic Acids. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim, Germany.
- Hazardous Substances Data Bank (HSDB).
- Rossberg M, Lendle W, Pfleiderer G, Tögel A, Dreher EL, et al. (2006) Chlorinated Hydrocarbons. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH, Weinheim, Germany.
- NCI Thesaurus a (NCIt a).
- Lewis RJ (2001) Hawley's Condensed Chemical Dictionary 14th John Wiley & Sons, Inc. New York, USA, pp. 472, 738, 927.
- Decaux G, Andres C, Gankam KF, Soupart A (2010) Treatment of euvolemic hyponatremia in the intensive care unit by urea. Crit Care14(5): R184.
- Zong Y, Wang J, Yue G, Feng L, Song Z, et al. (2005) Traceless liquid-phase synthesis of 3-alkylamino-4,5-disubstituted-1,2,4-triazoles on polyethylene glycol (PEG). Tetrahedron Letters 46(31): 5139-5141.
- Ohkubo M, Kawamoto H, Ohno T, Nakano M, Morishima H (1997) Synthesis of NB-506, a new anticancer agent. Tetrahedron 53(2): 585-592.
- NCI Thesaurus b.
- Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, et al. (2008) A new class of anthelmintics effective against drug-resistant nematodes. Nature 452(7184): 176-180.
- US Coast Guard (1999) Chemical Hazard Response Information System (CHRIS) - Hazardous Chemical Data. Commandant Instruction 16465.12C. Government Printing Office, Washington DC, USA.
- Yoshikawa M, Eckert JW, Keen NT (1976) The mechanism of fungistatic action of sec-butylamine: II. The effect of sec-butylamine on pyruvate oxidation by mitochondria of Penicillium digitatum and on the pyruvate dehydrogenase complex. Pestic Biochem Physiol 6(5): 482-490.
- Chemical Entities of Biological Interest b (ChEBI b).
- Prado Prado FJ, García Mera X, González Díaz H (2010) Multi-target spectral moment QSAR versus ANN for antiparasitic drugs against different parasite species. Bioorg Med Chem 18(6): 2225-2231.
- Kusumi T, Yoneda K, Kakisawa H (1979) A Convenient Synthesis of 5,6,7,8-Tetrahydroquinoline. Synthesis 221-221.
- Vishnu JR, Arun S, Mahendra N, Ramendra P (2019) Three-Membered Ring Heterocycles. In The Chemistry of Heterocycles pp: 19-92.
- Bartholomaeus A, Haritos V (2005) Review of the toxicology of carbonyl sulfide, a new grain fumigant. Food Chem Toxicol 43(12): 1687–1701.
- Harmeier A, Meyer CA, Staempfli A, Casagrande F, Petrinovic MM, et al. (2018) How Female Mice Attract Males: A Urinary Volatile Amine Activates a Trace Amine-Associated Receptor That Induces Male Sexual Interest. Front Pharmacol 9: 924.
- Human Metabolome Database (HMDB).
© 2021 Bhange DS. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






