- Submissions

Full Text
Annals of Chemical Science Research
Nimesulide Based 1,2,4,5-Tetra Substituted Imidazole Derivative: Synthesis and Characterisation
John Sunil R1*, Sai Kiran D1, Sridhar V1, Sarbani Pal2 and Jayashree A3
1Vaageswari Institute of Pharmaceutical Sciences, India
2MNR PG College, India
3IST, JNTUH, India
*Corresponding author: John Sunil R, Department of Chemical Sciences, Vaageswari Institute of Pharmaceutical Sciences, India
Submission: October 01, 2021;Published: October 21, 2021

Volume2 Issue5October, 2021
Chemical Abstract
Nimesulide a preferential ‘‘cyclooxygenase-2 inhibitor’’ is one of the well-known non-steroidal antiinflammatory drugs that has been utilized to treat pain and other inflammatory diseases. Nimesulide was withdrawn from the market due to its hepatotoxicity which could be due to the presence of nitro group. Imidazoles represent an important class of bioactive molecules that shows a wide range of pharmacological activities besides anti-inflammatory activity. In our strategy to develop safer and potential anti-inflammatory molecules, we have decided to combine some of the structural features of nimesulide and imidazole in a single molecule. We have described the design and synthesis of nimesulide based 1,2,4,5-tetra substituted imidazole of potential biological significance via chemical modifications of a commonly used anti-inflammatory agent nimesulide. This derivative was prepared from nimesulide via a two-step process involving regio selective reduction of nimesulide followed by hetero cyclisation of reduced nimesulide in very 81 % yield. The title compound nimesulide based 1,2,4,5-tetra substituted imidazole was synthesized in very good yield by reaction of benzil, benzaldehyde, ammonium acetate, and N-(4-amino-2-phenoxy phenyl) methane sulphonamide in acetic acid using multi-component strategy and molecular modification. The structure of the synthesized compound was confirmed by IR and H1 NMR spectral analysis.
Keywords:Nimesulide; Nimesulide based 1,2,4,5-tetra substituted imidazole; Molecular modification
Introduction
Nimesulide a preferential ‘‘cyclooxygenase-2 inhibitor’’ is one of the well-known non-steroidal anti-inflammatory drugs that have been utilized to treat pain and other inflammatory diseases. Nimesulide was withdrawn from the market due to its hepatotoxicity which was due to the presence of nitro group(toxicophore) [1,2]. Imidazoles represent an important class of bioactive molecules that shows a wide range of pharmacological activities besides anti-inflammatory activity. In our strategy to develop safer and potential antiinflammatory molecules, we have decided to modify the toxicophore and integrate some of the structural features of nimesulide and imidazole in a single molecule. Because of their common anti-inflammatory properties and our interest in nimesulide derivatives as potential anti-inflammatory agents, we decided to prepare a compound having structural features of both compounds. We estimated that a combination of structural features of imidazole with nimesulide in a single molecule would provide novel agents possessing potent pharmacological activities. We report the synthesis nimesulide based 1,2,4,5 -tetra substituted imidazole as hybrid molecule derived from nimesulide in very 81% yield from nimesulide via reducing its nitro group followed by hetero cyclisation using multicomponent strategy [3,4].
Synthetic scheme:
Figure 1:
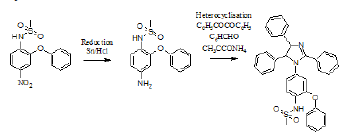
Synthesis of Nimesulide based 1,2,4,5-tetra substituted Imidazole
Materials and Methods
Synthetic procedure
0.016 moles of reduced nimesulide, 0.005 moles of benzil, 0.005 moles of benzaldehyde, 0.012moles of ammonium Acetate, 0.174 moles of acetic acid were taken in a clean, dry round bottom flask fitted with a reflux condenser along with condenser pipes. The reaction mixture was heated on a magnetic stirrer at 970 RPM with a hot plate to reflux at 120 °C for about 6 hours. The progress of the reaction was monitored using ascending TLC technique. Excess acetic acid was distilled off and the reaction mixture was quenched into 100mL of ice-cold water [5,6]. Crude 1,2,4,5 tetra substituted imidazole separated out as solid which was filtered at suction, washed with sodium bisulfite wash, cold water, dried, and recrystallized from suitable recrystallization technique.
Results and Discussion
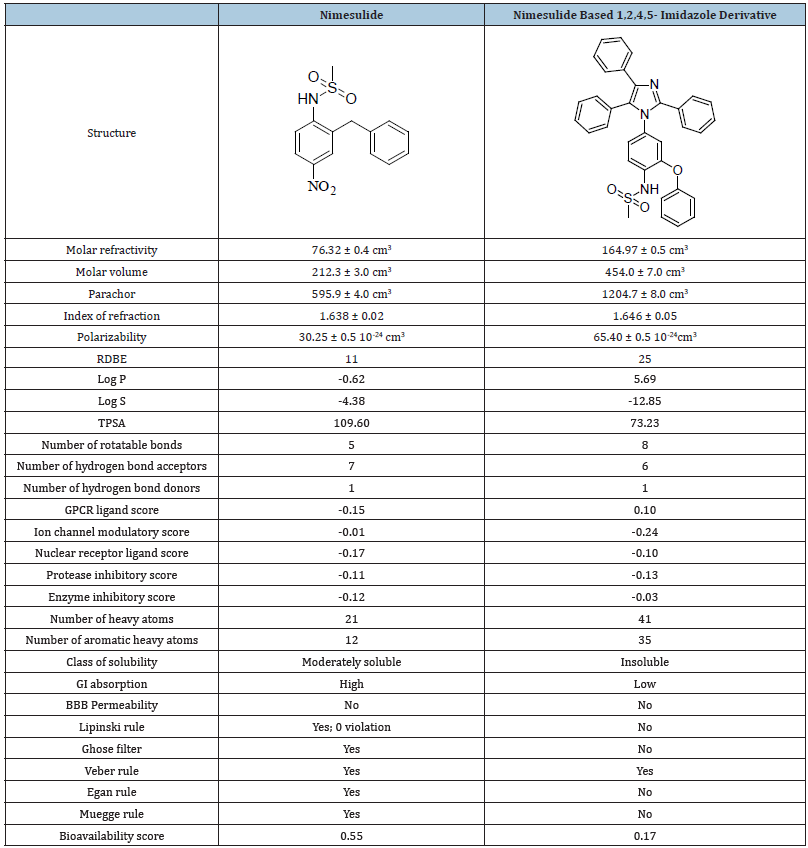
Computational data of the title compound using Chem sketch, Mol inspiration, Pro Tox-II and Swiss ADME (Table 1 & 2).
Table 1: Table of characterization.
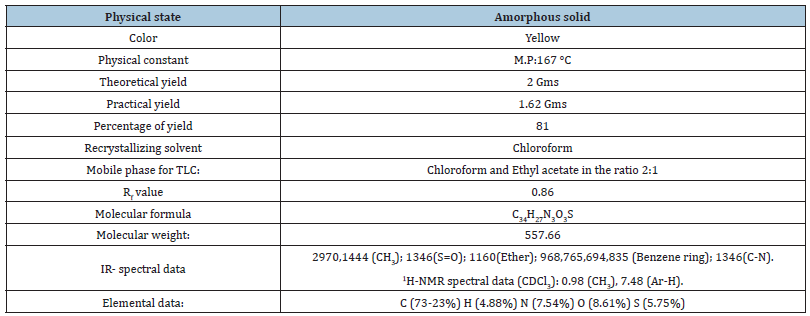
Table 2:Table of computational data.
Conclusion
In conclusion, we have described the design and synthesis of nimesulide based 1,2,4,5- tetra substituted imidazole of potential biological significance via chemical modifications of a commonly used anti-inflammatory agent nimesulide. It was prepared from Nimesulide via a two-step process involving reduction of nimesulide followed by hetero cyclisation of reduced nimesulide in very 81% yield. Moreover, because of the lack of nitro group in the final molecule, it is expected to be free from the side effects of nimesulide such as hepatotoxicity which is due to nitro group. Overall, the present nimesulide-based imidazole framework appeared to be a useful template for the design and identification of novel and potential anti-inflammatory agents.
Acknowledgments
The author thanks Mr. G. Sreenivas Reddy the chairman of Vaageswari educational trust for his constant encouragement and T. Ashritha mam for guiding us invitro Anti-inflammatory studies.
References
- John H Gajewski (1965) Molecular Modification in Drug Design. Clinical Chemistry 11(5): 612.
- Sandhya P, Jyoti M, Geetha Rani DP, Padmavathi VG, Sarbani P (2007) Chemical Modifications of Nimesulide. J Braz Chem Soc 18(2): 384-390.
- Ghodsi MZ, Zeinab D, Monireh SN, Alireza B (2015) One-pot synthesis of 1,2,4,5-tetra substituted Imidazoles using sulfonic acid functionalized silica (SiO2-Pr-SO3H). Arabian Journal of Chemistry 8(5): 692-697.
- Nascimento MVPS, Munhoz ACM, Theindl LC, Mohr ETB, Saleh N, et al. (2018) A novel tetrasubstituted imidazole as a prototype for the development of anti-inflammatory drugs. Inflammation 41(4): 1334-1348.
- Banerjee P, Eckert OA, Schrey AK, Preissner R (2018) ProTox-II: a webserver for the prediction of toxicity of chemicals. Nucleic Acids Res 46(W1): W257-W263.
- Antoine Daina, Olivier Michielin, Vincent Zoete (2017) Swiss ADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules 7: 42717.
© 2021 John Sunil R. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






