- Submissions

Full Text
Advances in Complementary & Alternative medicine
Medical Cannabis, Melatonin and Oxygen- Ozone as Integrative Therapies for the Treatment of Anaplastic Astrocytoma: A Case Report
Alessandra Mammone1, Paola Zuccoli2, Martina Giangrossi3, Maria Beatrice Morelli3, Alessandro Fanelli2, Ludovica Fedi4, Alfredo Longo4, Beatrice Pantolini5, Massimo Nabissi3 and Margherita Luongo4,6*
1Specialization School of Hospital Pharmacy, University of Perugia, Italy
2Department of Radiotherapy, Institute Ecomedica Empoli, Italy
3School of Pharmacy, University of Camerino, Italy
4Maria Guarino Foundation-AMOR No Profit Association, Italy
5SC Farmacia Aziendale, SC Medicina Interna, UOSD Dietologia e Nutrizione Clinica, Italy
6School of Medicine and Surgery, University of Campania “Luigi Vanvitelli”, Italy
*Corresponding author:Margherita Luongo, School of Medicine and Surgery, University of Campania “Luigi Vanvitelli”, Maria Guarino Foundation-AMOR No Profit Association, Pozzuoli, Naples, Italy
Submission: July 14, 2025;Published: September 09, 2025

ISSN: 2637-7802 Volume 8 Issue 5
Abstract
Astrocytoma is a brain tumor that is well known for its aggressiveness and limited survival rate. Standard medical treatments include chemotherapy, radiotherapy and surgery, but their use is associated with several long-term adverse effects. As a result, many patients are forced to interrupt their cure or to worsen their quality of life. Among the alternative therapies available to cancer patients, the combination of Melatonin (MLT), medical cannabis, and Oxygen-Ozone (O2O3) therapy has several advantages, both during and after the standard treatment course. In May 2017, after speech disorders and motor deficits in the right upper limb appeared in a 48-year-old Italian woman, Magnetic Resonance Imaging (MRI) was performed. An expansive lesion in the cortico-subcortical brain area was detected and an isocitrate dehydrogenase 1- wild-type- diffuse anaplastic astrocytoma was diagnosed. From September to November 2017, the patient underwent the first cycle of Radio Therapy (RT) and Chemo Therapy (CT). In January 2018, the patient started O2O3 therapy. In July 2018, therapy was implemented with MLT and medical cannabis. In December 2018, the patient completed CT and RT but continued receiving this integrative therapy. In February 2019, a pet scan revealed no metabolically active disease in the brain and follow-up magnetic resonance imaging revealed that the lesion remained stable. After six years of integrative treatments, subsequent magnetic resonance imaging confirmed the stability of the tumor mass. This case report highlights the beneficial effects and long-term acceptability of an adjuvant therapy of medical cannabis, MLT and O2O3 in a clinical context characterized by se-rious difficulties in approaching a standard protocol. Following several studies in which the anti-tumoral potentials of MLT, medical cannabis and O2O3 therapy have been observed separately, the positive results observed in this case report may represent another step toward obtaining more innovative and less debilitating therapeutic options.
Keywords:Anaplastic astrocytoma; Medical cannabis; Melatonin; Oxygen-ozone therapy; Case report
Introduction
Astrocytoma is an aggressive, hormone-sensitive brain tumor that originates from astrocytes [1]. It has an incidence rate of 0.48per 100,000 people [2] and it is characterized by poor diagnosis and limited survival rates, with a significantly higher incidence in females than in males (ratio 4:1) [1,3]. Starting in 2021, the World Health Organization (WHO) identified four types of astrocytomas, ranging from Grade I to Grade IV, based on genetic, morphological and clinical aspects [4]. Among these, Grade III, or anaplastic astrocytoma, is characterized by invasiveness and fast growth. The specific causes of its occurrence have not yet been clarified, but exposure to chemical agents or ionizing radiation or Mendelian disorders, including neurofibromatosis, tuberous sclerosis and Li-Fraumeni syndrome, is thought to play a significant role in astrocytoma emergence and development [1,5]. From a genetic perspective, anaplastic astrocytoma is characterized by genetic mutations, including mutations in isocitrate De Hydrogenase 1 (IDH1) enzymes, the telomere-binding protein Alpha Thalassemia/ Mental Retardation Syndrome X-Linked (ATRX) and the Tumor Suppressor Protein 53 (TP53) [6]. Interestingly, anaplastic astrocytoma with the IDH wild-type form is linked to a negative prognosis and TP56/ATRX mutations contribute to uncontrolled cellular proliferation and increased tumor aggressiveness [3,7].
Currently, the standard treatment protocol for patients with astrocytoma involves surgical resection when feasible, followed by Radiation Therapy (RT) and concurrent Chemotherapy (CT) using procarbazine, lomustine, vincristine, or Temozolomide (TMZ) [8]. Despite these established protocols, the treatment of anaplastic astrocytoma continues to pose significant clinical challenges, with a median survival time of 2-3 years [9]. Targeting approaches such as IDH1 or isocitrate De Hydrogenase 2 (IDH2) inhibitors (e.g., ipilimumab, nivolumab, and pembrolizumab) are being tested in clinical trials as new therapeutic options for treating astrocytoma [10]. Another important area of exploration involves the use of adjuvant therapies, including the protagonists described in this report. The Oxygen-Ozone (O₂O₃) therapy as integrative therapies in reduction of pain, improvement of physical function, reduction of inflammation and infection was reported [11]. It has several mechanisms of action that may support its inclusion in cancer therapies. First, O₂O₃ induces oxidative damage to cell membranes by targeting fatty acids and consequently, generating Reactive Oxygen Species (ROS), such as hydroperoxides and aldehydes. While antioxidant enzymes partially counteract this damage, insufficient Nicotinamide Adenine Dinucleotide Phosphate Hydrogen (NADPH), which is commonly observed in cancer, exacerbates oxidative stress, resulting in cellular damage and the activation of signaling pathways [12]. Second, O₂O₃ therapy induces the activation of antitumor T lymphocytes and natural killer cells, enhancing the efficacy of RT and chemotherapeutic agents [13]. Last, studies have suggested that O₂O₃ therapy can mitigate CT-induced side effects, such as nausea, hair loss and fatigue [14]. Melatonin (MLT), or N-acetyl-5-methoxytryptamine, is an endogenous hormone produced by the pineal gland in response to light [15]. As well documented by several studies, its therapeutic role is supported by its anticancer, immunostimulant and antioxidant properties [16-18]. Phyto cannabinoids, particularly Cannabidiol (CBD), are employed to treat various conditions, including epilepsy, cachexia associated with diseases such as AIDS and multiple sclerosis, anxiety and cancer-related symptoms [19]. Their role in inhibiting tumor progression has been extensively studied at the preclinical level, both in vitro and in vivo [20,21]. Additionally, evidence from phase Ib and phase II clinical trials involving the use of delta-9- Tetra Hydro Cannabinol (THC) and CBD further supports their therapeutic potential in managing high-grade gliomas [22,23]. Herein, we report the case of a 48-year-old woman diagnosed with IDH wild-type diffuse anaplastic astrocytoma. She underwent therapy with O₂O₃, medical cannabis and MLT during and after conventional therapy, yielding noteworthy outcomes. These results prompted us to share our findings and to further investigate this adjuvant treatment as a potential future strategy against cancer.
Case Presentation
In July 2017, a 48-year-old Italian woman after the sudden appearance of speech disorders and motor deficits in the right upper limb, underwent Magnetic Resonance Imaging (MRI), and an expansive lesion was detected in the cortico-subcortical area (Figure 1). The patient was a former smoker, with a family history of glioblastoma and lung cancer. The lesion was visible on T2 and FLAIR as signal hyperintensity, which then took up contrast medium. A stereotactic biopsy was subsequently performed, revealing millimetric fragments of brain tissue with morphological and immunophenotypic characteristics consistent with a grade 3 astrocytoma IDH-wild type (immunophenotype: GFAP+, IDH1-, EGFR-, p53 12‒13%, Ki67 <5%).From September to November 2017, the patient underwent brain RT via the intensity-modulated and Image-Guided Radiation Technique (IGRT/IMRT), and 54 Gy was delivered in 30 daily fractions of 1.8 Gy each. CT with TMZ was also performed at a dose of 120mg daily for 5 days each month over a period of 8 months. In January 2018, the patient initiated integrated O₂O₃ therapy while receiving CT and RT. The treatment protocol included rectal administration of O₂O₃ at a dose of 2.5mL/ kg, consisting of 97% oxygen and 3% ozone, at a concentration of 80μg/mL. This regimen was administered daily for four consecutive days each week. After completing three months of O₂O₃ therapy, the patient discontinued treatment for a period of five months.
The therapy was subsequently resumed and maintained to the present, with a reduction in frequency from four sessions per week to two, alternating between three months of active treatment and three months without therapy. During the check-up visits, monthly blood and biochemistry tests were carried out. At the July 2018 follow-up, the patient reported an overall sense of well-being during the O₂O₃ therapy cycles with a reduction in chemotherapy-related side effects. However, she experienced mild asthenia during breaks from O₂O₃ therapy. Compared with the beginning of the integrated treatment, the patient reported improvements in her speech and a reduction in bloating. In July 2018, integrative therapy with O2O3 was implemented with MLT and medical cannabis. MLT was initially set at a dosage of 100mg daily and was administered orally, with a gradual increase of 100mg every 4 days, reaching a maximum dose of 2g. The dosage was then stabilized and maintained at 2g per day. For medical cannabis, the patient initially received a dose of 50mg of Bedrolite (CBD 9%, Δ9-THC 0.4%) four times daily and 100mg of Bedrocan (Δ9-THC 22%, CBD <1.0%) four times daily. In December 2018, the patient completed chemotherapy and radiotherapy but continued receiving integrative therapy. Starting in 2019, the Bedrolite regimen was modified to 50mg twice daily during the O₂O₃ therapy pause phase and 30mg once daily during the O₂O₃ therapy period. On February 2019, a pet scan revealed no metabolically active disease in the brain and a follow-up MRI on May 2019 revealed no changes, with the lesion remaining stable, no enhancing and unchanged (Figure 2). Over five years of integrative treatments, subsequent MRI confirmed the stability of the tumor mass, including the most recent imaging on October 2024 and allowed the patient to be considered cured and able to lead a normal life (Figure 3).
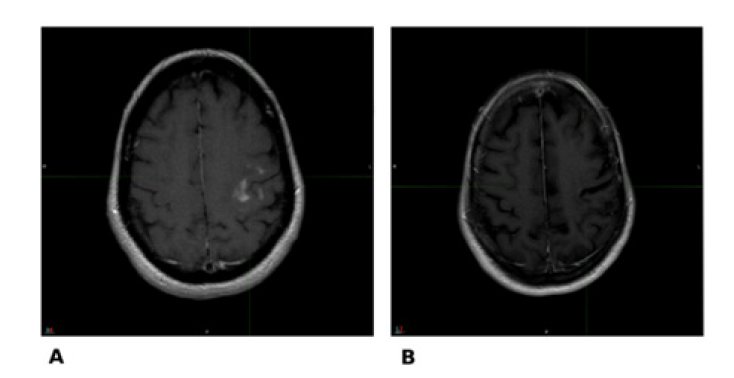
Figure 1:MRI performed for diagnosis (07/28/2017): (A) Axial; (B) Sagittal; (C) Coronal.
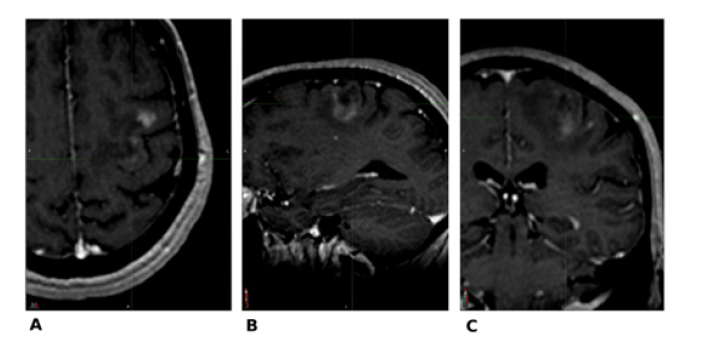
Figure 2:Comparative image between MRI dated 07/28/2017 (A axial and C sagittal) and follow-up MRI dated 05/22/2019 (B, axial e D, sagittal).
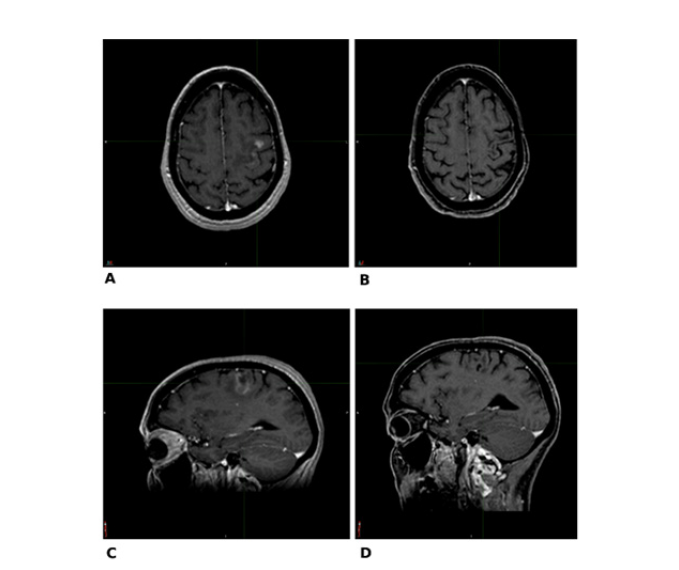
Figure 3:Comparative imaging between MRI dated 07/28/2017 (A) and last follow-up MRI dated 10/24/2024 (B).
Discussion
In this case report, a 48-year-old woman diagnosed with an anaplastic astrocytoma underwent adjuvant therapy consisting of MLT, medical cannabis and O₂O₃ therapy associated with standard RT and CT. Phyto cannabinoids, which are contained in medical cannabis, are derived from Cannabis sativa plants and Δ9-THC and CBD are the most prominent compounds. Several mechanisms are involved in the activity of these molecules, including acting as agonists or inverse agonists of cannabinoid receptors CB1 and CB2. Furthermore, cannabinoids target G protein-coupled receptors (GPCRs), including GPR12, GPR18, GPR35, GPR55, and GPR119, as well as opioid receptors, serotonin receptors, transient receptor potential vanilloid channels such as TRPV1 and TRPV2 [19]. Since 1975, Phyto cannabinoids have been widely investigated in different medical fields, obtaining interesting results in oncology. At first used to reduce CT side effects, including nausea, vomiting and cachexia [24], Phyto cannabinoids are also characterized by direct action on cancer cells [25].
With respect to brain tumors, particularly glioma cells, CBD reduces the expression and activity of Vascular Growth Factor Endothelial Receptor 2 (VEGFR-2), an important regulator of neovascularization in tumor [26]. A second mechanism for reducing the invasiveness of gliomas is the downregulation of Matrix Metalloproteinase-2 (MMP-2). [27,28] Last, Nabissi et al. [29]. suggested that CBD, through the activation of TRPV2, sensitizes Glioblastoma (GBM) cells to TMZ and BCNU increasing drug uptake and synergizing with these cytotoxic agents to induce apoptosis of glioma cells. Moreover, CBD is able to act on the tumor stem cell compartment promoting their differentiation both in vitro and in vivo mouse model [29]. The positive effects of cannabinoids observed in preclinical models have led to a placebo-controlled phase I clinical trial evaluating a cannabinoids combination alongside dose-intense TMZ in GBM patients (clinical trial NCT01812603). A total of 21 adult patients with GBM and a Karnofsky performance score of 60% or higher were randomized to take TMZ with placebo or nabiximols (a solution containing 27mg/ ml THC and 25mg/ml CBD). The results revealed that while the control group demonstrated a 44% one-year survival rate, the TMZ plus nabiximols group achieved an 83% one-year survival rate and a median survival of over 662 days [22]. Further advancement was achieved with the ARISTOCRAT: A phase II, multicenter, doubleblind, placebo-controlled, randomized trial [23].
This study involved a sample size of 234 patients randomized at a 2:1 ratio to receive either nabiximols or placebo in combination with standard TMZ treatment. Although data are still not available and the preliminary clinical findings cannot be deemed statistically significant owing to the small sample size, they further underline the importance of translational research on cannabinoids in the development of clinically effective therapies. In addition to CBD, the patient was supplemented with MLT during and after chemotherapy. MLT exhibits a range of antitumor effects, including antioxidant, cytostatic, antiproliferative, and proapoptotic activities, in addition to its ability to regulate epigenetic responses [30]. These properties have been investigated and documented in several studies on different cancer cells. Notably, Sung et al. [31]. demonstrated that MLT, with vorinostat (a histone deacetylase inhibitor), downregulates Transcription Factor EB (TFEB) and its oligomerization in GBM cells and glioma cancer stem cells. This suppression leads to increased apoptosis-related gene expression and promotes cell death. Another study by Fernandez-Gil et al. [32]. on GBM cells revealed the antiproliferative action of MLT, by increasing the metabolic switch from anaerobic glycolysis to an oxidative phosphorylation state in cancer cells. In colon cancer cells, MLT downregulates the Phosphatidylinositol 3-Kinase/Protein Kinase B (PI3K/AKT) and Nuclear Factor-kB/Inducible Nitric Oxide Synthase (NFkB/iNOS) signaling pathways, which are important regulators of differentiation, proliferation and cell survival [33]. Moreover, MLT synergistically affects the ability of 5-fluorouracil to inhibit Hypoxia-Inducible Factor 1 (HIF-1), a critical factor employed by cancer cells to survive under low-oxygen conditions [34].
In the clinical field, the association of MLT with standard chemotherapy has resulted in an increase in its efficacy, the mitigation of its side effects and an improvement in patient quality of life [35,36]. A meta-analysis by Seely et al. [37]. reported the beneficial use of oral MLT, alone and in combination with chemotherapy, in a substantial number of patients [37]. A total of 3697 patients from 21 clinical studies with different types of cancer revealed a pooled Relative Risk (RR) of 0.63 for one-year mortality (95% CI=0.53-0.74; p<0.001). Significant improvements were noted with MLT administration for complete response, partial response and stable disease, with RRs of 2.33, 1.90, and 1.51, respectively [37]. Moreover, a decrease in the incidence of alopecia, anemia, asthenia and thrombocytopenia associated with MLT administration was observed [37]. These findings strongly encourage the application of MLT in chemotherapy, even if further research is essential to elucidate its therapeutic potential. With respect to O₂O₃ therapy in cancer, only a limited number of clinical trials have investigated its use as an antitumor or palliative treatment. In vitro, with its several mechanisms of action, O₂O₃ notably reduces the growth of different types of cancer cell lines, including breast, lung, uterus and neuroblastoma cells [38,39]. A pivotal advancement in the investigation of this adjuvant therapy has emerged from studies by Luongo et al. [40]. on pancreatic ductal adenocarcinoma cell lines, which demonstrated the synergistic interaction between CBD and O₂O₃ therapy, both as a standalone treatment and in combination with antineoplastic agents (gemcitabine and paclitaxel). The combined use of MLT, CBD and O₂O₃ therapy has also been evaluated for the treatment of pancreatic ductal adenocarcinoma [41]. Both in vitro and in vivo experiments highlighted the anticancer effects of this combination. An increased response to standard chemotherapeutic agents commonly prescribed for the treatment of pancreatic ductal adenocarcinoma has also been reported, encouraging their use as an adjuvant therapy [41]. These findings underscore the therapeutic potential of this approach. It is also noted that expanded clinical data is required to validate these observations, as only one other case has reported the use of MLT, CBD and O2O3 therapy in the context of cancer therapy [42].
Conclusion
The data reported above and this patient experience support the rationale for using adjuvant therapy combining MLT, medical cannabis and O₂O₃. Significant benefits were observed both during conventional TMZ therapy and during the post treatment period. Notably, the patient demonstrated high compliance and a positive, proactive attitude toward the integrated treatment and no adverse effects or negative interactions with the ongoing oncological therapy were reported. Additionally, she opted to continue with MLT, medical cannabis and O₂O₃ therapy for several years, highlighting its potential long-term acceptability. Nevertheless, only one other case report has described the synergistic effects of this combination therapy, limiting our ability to confirm these findings conclusively. However, this case report contributes to the ongoing effort to fight against cancer and underscores the need for further investigation into innovative, less debilitating therapeutic options in oncology.
References
- Hirtz A, Rech F, DPSchneider H, Dumond H (2020) Astrocytoma: A hormone-sensitive tumor? Int J Mol Sci 21(23): 9114.
- Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, et al. (2022) Corrigendum to: CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2013-2017. Neuro-Oncol 24(7): 1214.
- Metu NCL, Sutihar SK, Sohel M, Zohora F, Hasan A, et al. (2023) Unraveling the signaling mechanism behind astrocytoma and possible therapeutics strategies: A comprehensive review. Cancer Rep Hoboken NJ 6(10): e1889.
- Bale TA, Rosenblum MK (2022) The 2021 who classification of tumors of the central nervous system: An update on pediatric low‐grade gliomas and glioneuronal tumors. Brain Pathol 32(4): e13060.
- Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, et al. (2014) The epidemiology of glioma in adults: A ‘state of the science’ review. Neuro Oncol 16(7): 896-913.
- Killela PJ, Pirozzi CJ, Reitman ZJ, Jones S, Rasheed BA, et al. (2014) The genetic landscape of anaplastic astrocytoma. Oncotarget 5(6): 1452-1457.
- Caccese M, Padovan M, Chioffi F, Gardiman MP, Berti F, et al. (2020) Anaplastic astrocytoma: State of the art and future directions. Crit Rev Oncol Hematol 153: 103062.
- Kessler T, Ito J, Wick W, Wick A (2023) Conventional and emerging treatments of astrocytomas and oligodendrogliomas. J Neurooncol 162(3): 471-478.
- Anami S, Fukai J, Hama M, Awaya A, Inagaki T, et al. (2021) Brainstem infiltration predicts survival in patients with high-grade gliomas treated with chemoradiotherapy. Anticancer Res 41(5): 2583-2589.
- Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH (2015) Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 11(9): 504-514.
- Serra MEG, Noci BJ, Abdala MCV, Luvisotto MM, Bertol CD, et al. (2023) The role of ozone treatment as integrative medicine. An evidence and gap map. Front Public Health 10: 1112296.
- Noci BJ, Bonilla PR (2021) Systemic review: Ozone: A potential new chemotherapy. Int J Mol Sci 22(21): 11796.
- Clavo B, Rodríguez SN, Llontop P, Gutiérrez D, Suárez G, et al. (2018) Ozone therapy as adjuvant for cancer treatment: Is further research warranted? Evid Based Complement Alternat Med 2018: 7931849.
- Luongo M, Brigida AL, Mascolo L, Gaudino G (2017) Possible therapeutic effects of ozone mixture on hypoxia in tumor development. Anticancer Res 37(2): 425-435.
- Claustrat B, Leston J (2015) Melatonin: Physiological effects in humans. Neurochirurgie 61(2-3): 77-84.
- Gitto E, Tan DX, Reiter RJ, Karbownik M, Manchester LC, et al. (2001) Individual and synergistic antioxidative actions of melatonin: Studies with vitamin E, vitamin C, glutathione and desferrrioxamine (desferoxamine) in rat liver homogenates. J Pharm Pharmacol 53(10): 1393-1401.
- Marques JHM, Mota AL, Oliveira JG, Lacerda JZ, Stefani JP, et al. (2018) Melatonin restrains angiogenic factors in triple-negative breast cancer by targeting miR-152-3p: In vivo and in vitro Life Sci 208: 131-138.
- Moradkhani F, Moloudizargari M, Fallah M, Asghari N, Heidari Khoei H, et al. (2020) Immunoregulatory role of melatonin in cancer. J Cell Physio ln 235(2): 745-757.
- Legare CA, Konsavage RWM, Vrana KE (2022) Therapeutic potential of cannabis, cannabidiol, and cannabinoid-based pharmaceuticals. Pharmacology 107(3-4): 131-149.
- Blázquez C, Feria GL, Álvarez L, Haro A, Casanova ML, et al. (2004) Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res 64(16): 5617-5623.
- Rocha FCM, Santos DJG, Stefano SC, Silveira DDX (2014) Systematic review of the literature on clinical and experimental trials on the antitumor effects of cannabinoids in gliomas. J Neurooncol 116(1): 11-24.
- Twelves C, Sabel M, Checketts D, Miller S, Tayo B, et al. (2021) A phase 1b randomised, placebo-controlled trial of nabiximols cannabinoid oromucosal spray with temozolomide in patients with recurrent glioblastoma. Br J Cancer 124(8): 1379-1387.
- Bhaskaran D, Savage J, Patel A, Collinson F, Mant R, et al. (2024) A randomised phase II trial of temozolomide with or without cannabinoids in patients with recurrent glioblastoma (ARISTOCRAT): protocol for a multi-center, double-blind, placebo-controlled trial. BMC Cancer 24(1): 83.
- Smith LA, Azariah F, Lavender VT, Stoner NS, Bettiol S (2015) Cannabinoids for nausea and vomiting in adults with cancer receiving chemotherapy. Cochrane Database Syst Rev 2015(11): CD009464.
- Cort SA, Sánchez MC, Espel E (2020) Anti-proliferative and cytotoxic effect of cannabidiol on human cancer cell lines in presence of serum. BMC Res Notes 13(1): 389.
- Kyriakou I, Yarandi N, Polycarpou E (2021) Efficacy of cannabinoids against glioblastoma multiforme: A systematic review. Phytomedicine 88: 153533.
- Blázquez C, Salazar M, Carracedo A, Lorente M, Egia A, et al. (2008) Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res 68(6): 1945-1952.
- Buchalska B, Kamińska K, Larsson OM, Jędrzejewska CA (2024) Cannabinoids in the treatment of glioblastoma. Pharmacol Rep 76(2): 223-234.
- Nabissi M, Morelli MB, Amantini C, Liberati S, Santoni M, et al. (2025) Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int J Cancer 137(8): 1855-1869.
- Favero G, Moretti E, Bonomini F, Reiter RJ, Rodella LF, et al. (2018) Promising antineoplastic actions of melatonin. Front Pharmacol 9: 1086.
- Sung GJ, Kim SH, Kwak S, Park S, Song J, et al. (2019) Inhibition of TFEB oligomerization by co‐treatment of melatonin with vorinostat promotes the therapeutic sensitivity in glioblastoma and glioma stem cells. J Pineal Res 66(3): e12556.
- Gil FBI, Lopez OA, Bechtle A, Ramos VCA, Qosja N, et al. (2022) Melatonin treatment triggers metabolic and intracellular ph imbalance in glioblastoma. Cells 11(21): 3467.
- Mota A, Perassi BJ, Castro TD, Colombo J, Sonehara N, et al. (2019) Melatonin modifies tumor hypoxia and metabolism by inhibiting HIF-1α and energy metabolic pathway in the in vitro and in vivo models of breast cancer. Melatonin Research 4: 83-98.
- Talib WH, Alsayed AR, Abuawad A, Daoud S, Mahmod AI (2021) Melatonin in cancer treatment: Current knowledge and future opportunities. Molecules 26(9): 2506.
- Liu D, Ma Z, Di S, Yang Y, Yang J, et al. (2018) AMPK/PGC1α activation by melatonin attenuates acute doxorubicin cardiotoxicity via alleviating mitochondrial oxidative damage and apoptosis. Free Radic Biol Med 219: 59-72.
- Kim C, Kim N, Joo H, Youm JB, Park WS, et al. (2005) Modulation by melatonin of the cardiotoxic and antitumor activities of adriamycin. J Cardiovasc Pharmacol 46(2): 200-210.
- Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, et al. (2012) Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. I Integr Cancer Ther 11(4): 293-303.
- Yıldırım M, Erkişi S, Yılmaz H, Ünsal N, İnaç E, et al. (2022) The apoptotic effect of ozone therapy on mitochondrial activity of highly metastatic breast cancer cell line MDA-MB-231 using in vitro J Interv Med 5(2): 64-71.
- Cannizzaro A, Falzacappa CV, Martinelli M, Misiti S, Brunetti E, et al. (2007) O2/3 exposure inhibits cell progression affecting cyclin B1/cdk1 activity in SK‐N‐SH while induces apoptosis in SK‐N‐DZ neuroblastoma cells. J Cell Physiol 213(1):115-125.
- Luongo M, Marinelli O, Zeppa L, Aguzzi C, Morelli MB, et al. (2025) Cannabidiol and oxygen-ozone combination induce cytotoxicity in human pancreatic ductal adenocarcinoma cell lines. Cancers 17(3): 365.
- Zeppa L, Aguzzi C, Morelli MB, Marinelli O, Amantini C, et al. (2024) In vitro and in vivo effects of melatonin-containing combinations in human pancreatic ductal adenocarcinoma. J Pineal Res 76(5): e12997.
- Antonini M, Aguzzi C, Fanelli A, Frassineti A, Zeppa L, et al. (2023) The Effects of a combination of medical cannabis, melatonin, and oxygen-ozone therapy on glioblastoma multiforme: A Case Report. Reports 6(2): 22.
© 2025 Margherita Luongo . This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






