- Submissions

Full Text
Advances in Complementary & Alternative medicine
Single Dose Administration of Valeriana officinalis Extract Improves Actual Sleep Time: A Randomized, Double-Blind, Placebo- Controlled Study
Harshith Chandra Shekhar1, Abhijeet Morde2, Muralidhara Padigaru2, Lincy Joshua3 and Jestin V Thomas3*
1BGS Global Institute of Medical Sciences, India
2OmniActive Health Technologies, India
3Leads Clinical Research and Bio Services Pvt. Ltd, India
*Corresponding author:Jestin V Thomas, Leads Clinical Research and Bio Services Pvt. Ltd. No.9, 1st Floor Mythri Legacy, Kalyan Nagar, Chelekere Main Road, Bengaluru 560043, Karnataka, India
Submission: October 23, 2024;Published: October 30, 2024

ISSN: 2637-7802 Volume 8 Issue 3
Abstract
Introduction: Insufficient sleep duration and poor sleep quality may negatively impact health. Valeriana
officinalis has been used traditionally to manage sleep-related issues. The objective of the study was to
evaluate the effect of V. officinalis Extract (VE) on sleep quality after single dose administration on day 1.
Methods: A randomized, double-blind, placebo-controlled, parallel, 8-week intervention study was
conducted to evaluate the effects of VE on sleep. Eighty subjects with sleep related complaints were
randomly assigned to VE and placebo groups in 1:1 ratio and were evaluated for effects on sleep
parameters like actual sleep time, sleep latency, and sleep efficiency using wrist actigraphy device.
Result: We evaluated data from 37 subjects in VE group and 35 from placebo group out of 80 subjects
randomized for the study. The VE group showed a significant increase (p<0.05) in actual sleep time as
compared to the placebo group on day 1 of the study. However, single dose administration of VE did not
show significant changes on sleep latency and sleep efficiency as compared to placebo.
Conclusion: Our study indicated significant improvement in the actual sleep time after a single dose
administration of VE suggesting an acute benefit of valerian on sleep. This acute effect of VE is significant
as we have observed additional sleep benefits on further supplementation including day 3 and 8 weeks
and thus potentially VE could be an alternative solution for those looking for early sleep benefits as a safe
alternative to melatonin.
Trial registration: Clinical trials registry of India: CTRI/2022/05/042818
Keywords:Sleep; Valerian; Sleeproot®; Anxiety; Melatonin
Abbreviations:AASM: American Academy of Sleep Medicine; BAI: Beck Anxiety Inventory; BMI: Body Mass Index; CTRI: Clinical Trials Registry of India; EEG: Electroencephalogram; GABA: Gamma- Aminobutyric Acid; HPLC: High-Performance Liquid Chromatography; PK: Pharmacokinetic; PSQI: Pittsburgh Sleep Quality Index; REM: Rapid Eye Movement; SE: Sleep Efficiency; VE: V. officinalis Extract
Introduction
Sleep deprivation and poor-quality sleep is a major evolving health concern world-wide [1,2]. Sleep negatively impacts quality of life and well-being particularly affecting metabolic and cognitive health [3-13]. It is believed that changing lifestyle associated with diet, and workload are contributing to poor quality sleep [14,15]. Melatonin is widely used supplement to improve sleep but [16] known to cause headache, sleepiness, light-headedness and vomiting sensations. In addition to regulation of body’s sleep-wake cycles, melatonin also affects number of biological system, including cardiovascular, reproductive, endocrine and metabolic systems and hence not recommended for long-term use [17]. Prescription drugs for sleep disorders that include benzodiazepines, anti-histamines etc., although used in clinics to address severe sleep disorders are not advisable for long term use due to multiple undesired adverse-effects [18-27]. Herbal supplements used extensively in traditional medicine are considered as an effective alternate for managing sleep due to their relative safety and lesser adverse effects [28,29]. Valeriana officinalis has been traditionally used to manage sleep across the globe [30-33].
Root extract of V. officinalis is known to have sedative, and anxiolytic effects, and improve overall sleep quality after oral intake [34]. Valerianic acid, which is the most common marker used for qualitative and quantitative analysis of valerian extract has been demonstrated to have sleep inducing properties [30,33,35]. Valerenic acid is known to bind A1 adenosine receptors [36], benzodiazepine receptors [37], as well as increase GABA levels in synaptic space [38]. In experimental sleep models in mice, VE enhanced sleep quality by increasing serum levels of serotonin, melatonin, and dopamine as well as increased expression of GABA receptors [39]. Further human clinical studies have demonstrated that valerian extract reduced sleep latency and improved sleep quality in healthy subjects as well as those suffering from sleep disorders [40-45]. We previously reported beneficial effects of the standardised V. officinalis Extract (VE) on overall sleep quality, sleep latency, sleep efficiency and actual sleep time, in subjects with mild insomnia with a significant decrease in sleep latency as early as three days of oral intake through an 8-weeks long randomized, double-blind, placebo-controlled, clinical study [46]. This study focuses on the acute effects of VE on sleep, utilizing wrist actigraphy data following a single dose of VE on the first day postsupplementation.
Study material
Valerian extract (Sleeproot®) was extracted from valerian roots using water: ethanol mixture followed by spray drying (OmniActive Health Technologies, Mumbai, India). The final formulation prepared as a powder contained 26.23% of extract and 73.27% of cellulose polymer (novo excipients, Navi Mumbai, India) and 0.5%, of colloidal silicon dioxide (Daksh Medicare, Mumbai, India) with a final concentration of 2% total valerenic acid as established by high performance liquid chromatography [46].
Study design and procedures
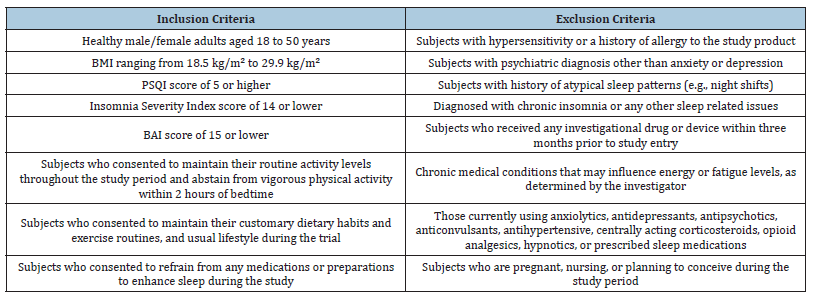
This was a randomized, double-blind, placebo-controlled, parallel, supplementation study done over 56 days including individuals with mild insomnia problems. Study was approved by ethics committee at BGS Global Institute of Medical Sciences, Bengaluru, India and registered at Clinical Trials Registry of India (CTRI/2022/05/042818). We followed ethical and regulatory guidelines from Indian Council of Medical Research, International Council for Harmonization Guidance on Good Clinical Practice (E6R2), and the Declaration of Helsinki. Eligible candidates provided written informed consent prior to enrolment to the study (Inclusion and exclusion criteria used for subject selection is provided in Table 1). Subjects were randomly divided at 1:1 ratio (using R software version 4.2.1 through an independent non-study expert) and informed to consume one capsule that contained either VE (200mg) or placebo (microcrystalline cellulose) every night, one hour before sleep for 56 days.
Methods
Table 1:Key inclusion/exclusion criteria.
Efficacy parameters
Participants visited the study centre in the night for recording sleep latency, actual sleep time and sleep efficiency using wrist actigraphy device on day 1.
Wrist actigraphy: Actigraphy devices are worn on the wrist which record movements to calculate sleep characteristics using specialized algorithms derived from computer software applications. The participants underwent wrist actigraphy evaluation at the research location and were instructed to wear Wrist actigraphy device (motionwatch 8, CamNtech Ltd. Cambridgeshire, UK) after dinner on day 1.
Sample size calculation and statistical tests
In order to determine statistically significant clinical difference between VE and placebo groups with 90% power and a 5% significance, 80 subjects (40 per group) were recruited for the study including the consideration of drop-out rate of 10% during the study. R software version 4.2.1 was used for statistical analysis using mean, standard error, 95% confidence interval in case of normal distribution of data and median if data was not normally distributed. Efficacy was assessed using actual values and mean changes from baseline and within-group analysis was done using paired t-test. Intergroup analysis was conducted using independent t-test and p-value of less than 0.05 was considered statistically significant.
Efficacy endpoints evaluation: Sleep parameters such as actual sleep time, sleep latency, and sleep efficiency were measured using wrist actigraphy from baseline to end of day 1.
Result
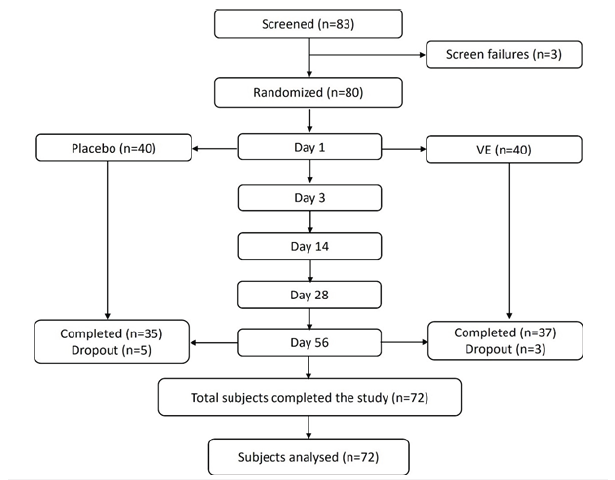
Out of 80 randomized subjects for the study, 35 in the placebo and 37 in the VE group completed the study and hence used for efficacy assessment (Figure 1). Five participants in the placebo group and three in the VE group were lost to follow-up and excluded from the study. The demographic details of the subjects who were included in the assessment are provided in the Table 2.
Table 2:Baseline demographics summary of study participants. N-Number of subjects in the specified treatment; n-number of subjects in the specified category; SE-Standard Error. Percentages are based on the number of subjects in the specified treatment.
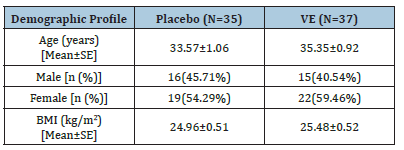
Figure 1:Consort diagram.
Efficacy results
A summary of Wrist Actigraphy results are provided in Table 3 and Figure 2.
Figure 2:Summary of acute effects on day 1 from wrist actigraphy results.
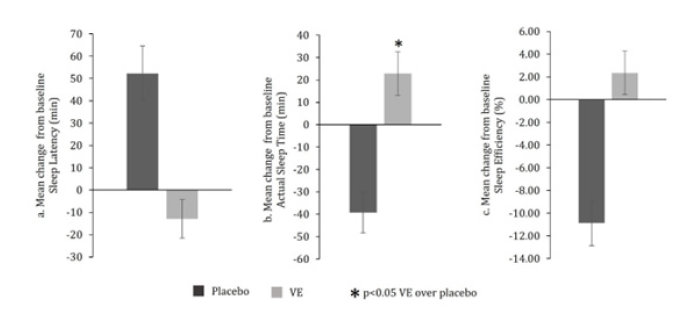
Table 3:Summary of results assessed by wrist actigraphy by treatment and visit. VE -Valerian extract; N-No of subjects; SE-Standard error; Min-Minimum; Max- Maximum; Change=Timepoint vs. Baseline; ##-Paired t-test (Timepoint vs. Baseline); ‡-Independent t-test; *–p<0.05 Valerian over placebo; #- p<0.1 & p>0.05 VE over placebo.
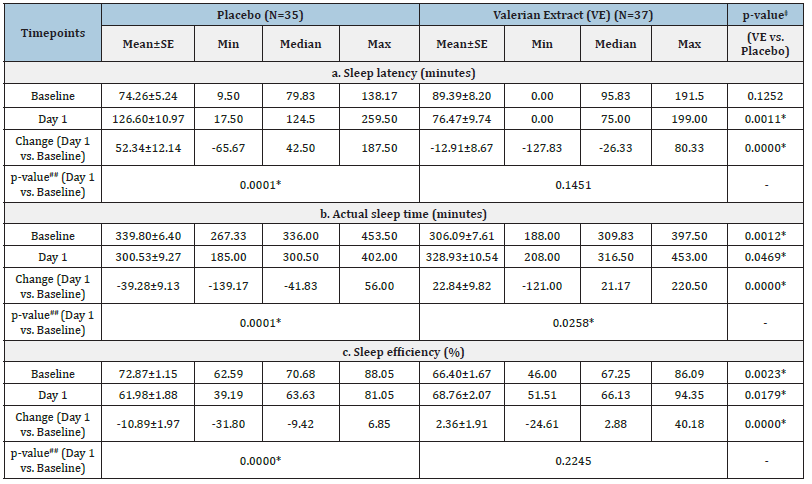
Sleep latency (minutes) as assessed by wrist actigraphy: In the VE group, a non-significant (p>0.05) decrease in the sleep latency (in minutes) as assessed by Wrist Actigraphy was recorded from 89.39±8.20 minutes at baseline to 76.47±9.74 minutes on day 1 whereas a significant (p<0.05) increase in sleep latency was observed in case of placebo from 74.26±5.24 minutes at baseline to 126.60±10.97 minutes on day 1 (Table 3a). The between group analysis showed a significant (p<0.05) difference in sleep latency between VE and placebo groups on day 1 (-12.91±8.67 minutes for VE vs. 52.34±12.14 minutes for placebo). However, this significance was not attributed to VE as there was no statistical significance in VE group at day 1 compared to baseline (Table 3a & Figure 2a).
Actual sleep time: In the VE group, a significant (p<0.05) increase in the actual sleep time (in minutes) as assessed by wrist actigraphy was recorded from 306.09±7.61 minutes at baseline to 328.93±10.54 minutes at day 1. On the contrary, placebo group showed a significant (p<0.05) decrease in the actual sleep time (in minutes) as assessed by wrist actigraphy from 339.80±6.40 minutes at baseline to 300.53±9.27 minutes at day 1 (Table 3b). Based on the between group analysis, VE group showed a significant (p<0.05) increase in the actual sleep time as compared to placebo from baseline on day 1 (22.84 ±9.82 minutes for VE vs. -39.28 ±9.13 minutes for placebo) (Table 3b & Figure 2b).
Sleep efficiency (%) as assessed by wrist actigraphy: In the VE group, a non-significant (p>0.05) increase in the sleep efficiency (%) as assessed by wrist actigraphy was recorded from 66.40±1.67 % at baseline to 68.76±2.07 % at day 1. In contrast, the placebo group demonstrated a significant (p<0.05) decrease in the Sleep efficiency (%) as assessed by wrist actigraphy from 72.87±1.15 % at baseline to 61.98±1.88 % on day 1 (Table 3c). The between group analysis showed a significant (p<0.05) difference in sleep efficiency between VE and placebo groups on day 1 (+2.36±1.91 % for VE vs. -10.89±1.97 % for placebo). However, this significance was not attributed to VE as there was no statistical significance in VE group at day 1 compared to baseline (Table 3c and Figure 2c).
Discussion
Sleep deficit impacts quality of life, cognitive performances, and increases risk of metabolic diseases [35,47-51]. Valerian extract has been widely used for improving sleep since antiquity [52]. Supplementation with VE has already been demonstrated to provide sleep benefits on various aspects of sleep by day 3 and several later time points during the course of 56 day study in subjects with mild insomnia [46]. Here we report acute effect of VE with significant improvement in the actual sleep time after a single oral dose of VE. We believe our observation in improvement of actual sleep time after single dose of standardized hydro-alcoholic extract of valerian is significant as it is an extract optimized to contain highest concentration of total valerenic acid (2%) reported to date with already reported sleep benefits after multiple doses of supplementation [46].
Further, the study material was found to be safe with no product related adverse events throughout the study period. Variety of valerian preparations and dosages that were evaluated in the past using various study designs, improved subjective experiences of sleep such as shortened sleep latency when ingested before sleep. In the past, clinical studies have used valerian extracts prepared as hydro-alcoholic extracts [53-60], aqueous extract [45,61,62], extracts prepared using unspecified solvents [63-65] or extracts defined as herbal substance (the whole root / rhizome) [66-70] to demonstrate sleep benefits. Interestingly, aqueous extracts of valerian found to provide sleep benefits after single dose such as improved subjective sleep quality in healthy volunteers [61,62]. Similarly, aqueous valerian extract provided dose-dependent increase in sleep latency as measured through wrist actigraphy at a dose of 450- or 900mg [62]. On the other hand, another study reported no sleep benefits as measured by polysomnography in subjects with insomnia after a single dose of valerian extract [53]. Further, other studies that used single dose of aqueous [45] or hydro-alcoholic [53,56] extract observed no sleep benefits although improved REM sleep was observed in insomnia patients [54]. Such inconsistent outcomes were observed also with repeated administration of hydro-alcoholic extracts [53,55,58-60,63]. There were 3 studies using extracts with unspecified procedures which showed inconsistent results with negative outcomes for 2 studies [64,65] and positive for an observational study [71].
On the other hand, all the 5 studies that used dried root/ rhizome of valerian reported improved sleep after single dose of supplementation [62,66-69] suggesting maximum efficacy from the total extracts of root / rhizome. It is believed that variation in sleep outcome across different studies of valerian supplementation may be due to the nature of preparation and type of extraction solvent used which in turn determines the chemical content of the extract [33,72,73]. This is further complicated by lack of consistency across studies with regard to study methods including randomization, criteria used for subject selection and variation in statistical methods used for data analysis [42,44,61,62,74]. It was also observed that effect of valerian extract on sleep was most significant in older male patients who considered themselves to be poor sleepers with lengthy sleep latencies as compared to those habitually good sleepers [62]. The valerian that was used in the various past studies used poorly defined extracts or extract that contained an amount of valerenic acid comparable to the 0.8% standard and lack of sedative effect noted in these studies was probably due to a lack of sufficient quantities of pharmacologically active molecules in the extract [75].
In our study we used well defined valerian extract with 2% total valerenic acid formulated and spray dried using excipients that are known to improve bioavailability as well as demonstrated to improve organoleptic properties (data not shown). Thus, the improved actual sleep time observed after single dose supplementation in our study is more meaningful and additional future studies should focus on validation of these data with larger set of subject population using multiple data evaluation methods in future. Wrist actigraphy is a valuable tool for assessment of sleep over extended periods of time in the natural sleep environment. With advancement of device technology and scoring algorithms wrist actigraphy is increasingly being used under clinical settings to measure sleep which is also substantiated by American Academy of Sleep Medicine (AASM). Further multiple studies that conducted comparative evaluation of wrist actigraphy to other established methods of assessing sleep has concluded that wrist actigraphy is useful for estimation of total sleep time, sleep percentage, and wake after sleep onset [76].
Motion Watch device used in the current study is one of the leading actigraphy solutions for convenient long-term sleep monitoring with high patient compliance and extensively used in clinical and scientific research applications [77]. We observed a 9% improvement in actual sleep time over baseline in our study. This is an important finding as the actual sleep time is crucial to improve brain performance, mood and overall health. In addition, VE supplementation showed marginal non-significant improvements in sleep latency (8.49%) and sleep efficiency (4.69%) compared to baseline, whereas subjects in the placebo group showed significant worsening of these parameters. This could be due to the change in sleep environment of subjects as they were housed at the study facility, a different environment than their regular sleep environment at home. However, it is important to note that even in this situation, VE supplementation showed significant effects on actual sleep time and slightly improved sleep latency and sleep efficiency which could be a point of further research in future studies. It is generally recommended that valerian be taken approximately 30 minutes to 2 hours prior to bedtime [53] which is further supported by human pharmacokinetic (PK) data with maximum serum concentration for valerenic acid achieved between 1 and 2 hours after single oral administration of valerian extract.
The PK study while explains the possible early effect of valerian extract seen in our study due to the presence of plasma level of valerenic acid within an hour and also indicate absence of adverse effects of valerian such as residual sedation in the morning [78]. We believe that our study outcome was limited by type of subject recruited for the study. Our subjects possibly had mild to moderate insomnia as against our targeted subjects with mild insomnia which was apparent from higher sleep latency observed at baseline. Additionally, no elderly subjects were included in the study. A study involving elderly people may provide more insights on the early effects of VE on sleep time across all age groups. Further we measured limited evaluation parameters at the end of single dose administration using only wrist actigraphy as most of the other parameters were measured at later time points of supplementation as reported in our previous studies [46]. Future detailed studies using subjective and objective parameters should provide more conclusive outcome of sleep benefits for our optimized hydroalcoholic preparation of valerian extract that is standardized to 2% total valerenic acid content.
Conclusion
Valerian is one of the most promising sleep-aid solutions today not only for improving quality of sleep but also established safety as a widely used solution in clinic and traditional medicine. Valerian extract 200mg (Sleeproot®) containing 2% total valerenic acid showed a significant improvement in the actual sleep time after a single dose which is a significant health benefit for those looking for early sleep benefits as a safe alternative to melatonin.
Acknowledgment
We thank the participants of the study. Disclosures. The study was funded by OmniActive Health Technologies. Abhijeet Morde and Muralidhara Padigaru are employees of OmniActive Health Technologies. The remaining authors state that the research was done in the absence of any business or financial relationships that may be considered a possible conflict of interest.
References
- Daley M, Morin CM, Blanc LM, Grégoire JP, Savard J (2009) The economic burden of Insomnia: Direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 32(1): 55-64.
- Wickwire EM, Shaya FT, Scharf SM (2016) Health economics of insomnia treatments: The return on investment for a good night’s sleep. Sleep Medicine Reviews 30: 72-82.
- Bonnet MH (1985) Effect of sleep disruption on sleep, performance, and mood. Sleep 8(1): 11-19.
- Dinges DF, Douglas SD, Hamarman S, Zaugg L, Kapoor S (1995) Sleep deprivation and human immune function. Advances in Neuroimmunology 5(2): 97-110.
- Knutson KL, Ryden AM, Mander BA, Cauter VE (2006) Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Archives of Internal Medicine 166(16): 1768-1774.
- Kasasbeh E, Chi DS, Krishnaswamy G (2006) Inflammatory aspects of sleep apnea and their cardiovascular consequences. Southern Medical Journal 99(1): 58-67.
- Taheri S (2006) The link between short sleep duration and obesity: We should recommend more sleep to prevent obesity. Archives of Disease in Childhood 91(11): 881-884.
- Schwartz DJ, Kohler WC, Karatinos G (2005) Symptoms of depression in individuals with obstructive sleep apnea may be amenable to treatment with continuous positive airway pressure. Chest 128(3): 1304-1309.
- Zimmerman M, Glinchey MJB, Young D, Chelminski I (2006) Diagnosing major depressive disorder I: A psychometric evaluation of the DSM-IV symptom criteria. The Journal of Nervous and Mental Disease 194(3): 158-163.
- Makarem N, Shechter A, Carnethon MR, Mullington JM, Hall MH, et al. (2019) Sleep duration and blood pressure: Recent advances and future directions. Current Hypertension Reports 21(5): 33.
- Hillman DR, Murphy AS, Antic R, Pezzullo L (2006) The economic cost of sleep disorders. Sleep 29(3): 299-305.
- Leger D (1994) The cost of sleep-related accidents: A report for the national commission on sleep disorders research. Sleep 17(1): 84-93.
- Stoller MK (1994) Economic effects of insomnia. Clinical Therapeutics 16(5): 873-897.
- Gall VC, Muth T, Angerer P (2023) Sleep duration on workdays is correlated with subjective workload and subjective impact of high workload on sleep in young healthy adults. Brain Sciences 13(5): 818.
- Kim H, Jeong G, Park YK, Kang SW (2018) Sleep quality and nutritional intake in subjects with sleep issues according to perceived stress levels. Journal of Lifestyle Medicine 8(1): 42-49.
- Oda FE, Qawasmi A, Bloch MH (2013) Meta-analysis: Melatonin for the treatment of primary sleep disorders. PloS One 8(5): e63773.
- Boutin JA, Kennaway DJ, Jockers R (2023) Melatonin: Facts, extrapolations and clinical trials. Biomolecules 13(6): 943.
- Schroeck JL, Ford J, Conway EL, Kurtzhalts KE, Gee ME, et al. (2016) Review of safety and efficacy of sleep medicines in older adults. Clinical Therapeutics 38(11): 2340-2372.
- Longo LP, Johnson B (2000) Addiction: Part I. Benzodiazepines-side effects, abuse risk and alternatives. American Family Physician 61(7): 2121-2128.
- Stewart SA (2005) The effects of benzodiazepines on cognition. Journal of Clinical Psychiatry 66(Suppl 2): 9-13.
- Hanson RC, Braselton JP, Hayes DC, Snyder RW, White JB, et al. (1986) Cardiovascular and renal effects of buspirone in several animal models. General Pharmacology 17(3): 267-274.
- Arendt J (1997) Safety of melatonin in long-term use (?). Journal of Biological Rhythms 12(6): 673-681.
- Händel MN, Andersen HK, Ussing A, Virring A, Jennum P, et al. (2023) The short-term and long-term adverse effects of melatonin treatment in children and adolescents: A systematic review and GRADE assessment. EClinicalMedicine 61: 102083.
- Olson LG (2008) Hypnotic hazards: Adverse effects of zolpidem and other z-drugs. Australian Prescriber 31: 146-149.
- Mets MAJ, Berend O, Volkerts ER, Verster JC (2008) Effects of hypnotic drugs on body balance and standing steadiness. Sleep Med Rev 14(4): 259-267.
- Vermeeren A, Coenen AM (2011) Effects of the use of hypnotics on cognition. Progress in Brain Research 190: 89-103.
- Bixler EO, Kales A, Brubaker BH, Kales JD (1987) Adverse reactions to benzodiazepine hypnotics: Spontaneous reporting system. Pharmacology 35(5): 286-300.
- Gooneratne NS (2008) Complementary and alternative medicine for sleep disturbances in older adults. Clinics in Geriatric Medicine 24(1): 121-138.
- Antoniades J, Jones K, Hassed C, Piterman L (2012) Sleep… naturally: A review of the efficacy of herbal remedies for managing insomnia. Alternative and Complementary Therapies 18(3): 136-140.
- Houghton PJ (1988) The biological activity of valerian and related plants. Journal of Ethnopharmacology 22(2): 121-42.
- Hobbs C (1989) Valerian: A literature review. 19-34.
- Barnes PM, Griner PE, McFann K, Nahin RL (2004) Complementary and alternative medicine use among adults: United States, 2002. Seminars in Integrative Medicine (343): 1-19.
- Houghton PJ (1999) The scientific basis for the reputed activity of valerian. Journal of Pharmacy and Pharmacology 51(5): 505-512.
- Nandhini S, Narayanan KB, Ilango K (2018) Valeriana officinalis: A review of its traditional uses, phytochemistry and pharmacology. Asian J Pharm Clin Res 11(1): 36-41.
- Pilerood SA, Prakash J (2013) Nutritional and medicinal properties of valerian (Valeriana officinalis) herb: A review. Int J Food Sci Nutr Diet 1(1): 25-33.
- Schumacher B, Scholle S, Hölzl J, Khudeir N, Hess S, et al. (2002) Lignans isolated from valerian: Identification and characterization of a new olivil derivative with partial agonistic activity at A(1) adenosine receptors. Journal of Natural Products 65(10): 1479-1485.
- Ortiz JG, Natal NJ, Chavez P (1999) Effects of Valeriana officinalis extracts on [3H] flunitrazepam binding, synaptosomal [3H] GABA uptake, and hippocampal [3H] GABA release. Neurochemical Research 24(11): 1373-1378.
- Santos M, Ferreira F, Cunha A, Carvalho A, Macedo T (1994) An aqueous extract of valerian influences the transport of GABA in synaptosomes. Planta Medica 60(3): 278-279.
- Sahin K, Gencoglu H, Korkusuz AK, Orhan C, Aldatmaz IE. et al. (2024) Impact of a novel valerian extract on sleep quality, relaxation, and GABA/serotonin receptor activity in a murine model. Antioxidants 13(6): 657.
- Morin CM, Culbert JP, Schwartz SM (1994) Nonpharmacological interventions for insomnia: A meta-analysis of treatment efficacy. Am J Psychiatry 151(8): 1172-1180.
- Bent S, Padula A, Moore D, Patterson M, Mehling W (2006) Valerian for sleep: A systematic review and meta-analysis. The American Journal of Medicine 119(12): 1005-1012.
- Leathwood PD, Chauffard F (1985) Aqueous extract of valerian reduces latency to fall asleep in man. Planta Medica 51(2): 144-148.
- Lindahl O, Lindwall L (1989) Double blind study of a valerian preparation. Pharmacology Biochemistry and Behavior 32(4): 1065-1066.
- Leathwood PD, Chauffard F (1982) Quantifying the effects of mild sedatives. Journal of Psychiatric Research 17(2): 115-122.
- Schulz H, Stolz C, Müller J (1994) The effect of valerian extract on sleep polygraphy in poor sleepers: A pilot study. Pharmacopsychiatry 27(4): 147-151.
- Shekhar CH, Joshua L, Thomas JV (2024) Standardized extract of Valeriana officinalis improves overall sleep quality in human subjects with sleep complaints: A randomized, double-blind, placebo-controlled, clinical study. Advances in Therapy 41(1): 246-261.
- Becker NB, Martins RIS, Jesus SDN, Chiodelli R, Rieber MS (2018) Sleep health assessment: A scale validation. Psychiatry Research 259: 51-55.
- Hoey LM, Fulbrook P, Douglas JA (2014) Sleep assessment of hospitalised patients: A literature review. International Journal of Nursing Studies 51(9): 1281-1288.
- Ibáñez V, Silva J, Cauli O (2018) A survey on sleep assessment methods. PeerJ 6: e4849.
- Sadeh A (2011) The role and validity of actigraphy in sleep medicine: An update. Sleep Medicine Reviews 15(4): 259-267.
- Colten HR, Altevogt BM (2006) Extent and health consequences of chronic sleep loss and sleep disorders. sleep disorders and sleep deprivation: An unmet public health problem. National Academies Press, USA.
- Martín FSMI, Font MR, Soler PL, Gómez SP, Caldentey CC, et al. (2010) Effectiveness of valerian on insomnia: A meta-analysis of randomized placebo-controlled trials. Sleep Medicine 11(6): 505-511.
- Donath F, Quispe S, Diefenbach K, Maurer A, Fietze I, et al. (2000) Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry 33(2): 47-53.
- Arellano HA, Villegas LG, Uriostegui CLM, Alvarez L, Pineda VG, et al. (2001) Polysomnographic evaluation of the hypnotic effect of Valeriana edulis standardized extract in patients suffering from insomnia. Planta Medica 67(8): 695-699.
- Ziegler G, Ploch M, Baumann MA, Collet W (2002) Efficacy and tolerability of valerian extract LI 156 compared with oxazepam in the treatment of non-organic insomnia-a randomized, double-blind, comparative clinical study. European Journal of Medical Research 7(11): 480-486.
- Diaper A, Hindmarch I (2004) A double‐blind, placebo‐controlled investigation of the effects of two doses of a valerian preparation on the sleep, cognitive and psychomotor function of sleep‐disturbed older adults. Phytotherapy Research 18(10): 831-836.
- Oxman AD, Flottorp S, Håvelsrud K, Fretheim A, Jensen OJ, et al. (2007) A televised, web-based randomised trial of an herbal remedy (valerian) for insomnia. PLoS One 2(10): e1040.
- Taibi DM, Vitiello MV, Barsness S, Elmer GW, Anderson GD, et al. (2009) A randomized clinical trial of valerian fails to improve self-reported, polysomnographic, and actigraphic sleep in older women with insomnia. Sleep Medicine 10(3): 319-328.
- Taibi DM, Bourguignon C, Taylor GA (2009) A feasibility study of valerian extract for sleep disturbance in person with arthritis. Biological Research for Nursing 10(4): 409-417.
- Koetter U, Schrader E, Käufeler R, Brattström A (2007) A randomized, double blind, placebo‐controlled, prospective clinical study to demonstrate clinical efficacy of a fixed valerian hops extract combination (Ze 91019) in patients suffering from non‐organic sleep disorder. Phytotherapy Research 21(9): 847-851.
- Balderer G, Borbély AA (1985) Effect of valerian on human sleep. Psychopharmacology 87(4): 406-409.
- Leathwood PD, Chauffard F, Heck E, Box MR (1982) Aqueous extract of valerian root (Valeriana officinalis) improves sleep quality in man. Pharmacology Biochemistry and Behavior 17(1): 65-71.
- Kuhlmann J, Berger W, Podzuweit H, Schmidt U (1999) The influence of valerian treatment on’reaction time, alertness and concentration’in volunteers. Pharmacopsychiatry 32(6): 235-241.
- Coxeter PD, Schluter PJ, Eastwood HL, Nikles CJ, Glasziou PP (2003) Valerian does not appear to reduce symptoms for patients with chronic insomnia in general practice using a series of randomised n-of-1 trials. Complementary Therapies in Medicine 11(4): 215-222.
- Jacobs BP, Bent S, Tice JA, Blackwell T, Cummings SR (2005) An internet-based randomized, placebo-controlled trial of kava and valerian for anxiety and insomnia. Medicine 84(4): 197-207.
- Cuellar NG, Ratcliffe SJ (2009) Does valerian improve sleepiness and symptom severity in people with restless legs syndrome? Alternative Therapies in Health & Medicine 15(2): 22-28.
- Ahmadi M, Khalili H, Abbasian L, Ghaeli P (2017) Effect of valerian in preventing neuropsychiatric adverse effects of efavirenz in HIV-positive patients: A pilot randomized, placebo-controlled clinical trial. Annals of Pharmacotherapy 51(6): 457-464.
- Barton DL, Atherton PJ, Bauer BA, Moore DF, Mattar BI, et al. (2011) The use of Valeriana officinalis (valerian) in improving sleep in patients who are undergoing treatment for cancer: A phase III randomized, placebo-controlled, double-blind study: NCCTG trial, N01C5. The Journal of Supportive Oncology 9(1): 24-31.
- Taavoni S, Ekbatani N, Kashaniyan M, Haghani H (2011) Effect of valerian on sleep quality in postmenopausal women: A randomized placebo-controlled clinical trial. Menopause 18(9): 951-955.
- Dominguez RA, Valverde BRL, Kaplowitz BR, Cott JM (2000) Valerian as a hypnotic for hispanic patients. Cultural Diversity and Ethnic Minority Psychology 6(1): 84-92.
- Wheatley D (2001) Kava and valerian in the treatment of stress‐induced insomnia. Phytotherapy Research 15(6): 549-551.
- Bisset NG, Wichtl M (1994) Herbal drugs and phytopharmaceuticals. Medpharm GmbH Scientific Publishers, Stuttgart, CRC Press, Boca Raton, USA, pp. 91-95.
- Hendriks H, Bos R, Woerdenbag H, Koster A (1985) Central nervous depressant activity of valerenic acid in the mouse. Planta Medica 51(1): 28-31.
- Gessner B, Klasser M (1984) Studies on the effect of harmonicum much on sleep using polygraphic EEG recordings. EEG EMG Zeitschrift Fur Elektroenzephalographie, Elektromyographie Und Verwandte Gebiete 15(1): 45-51.
- Glass JR, Sproule BA, Herrmann N, Streiner D, Busto UE (2003) Acute pharmacological effects of temazepam, diphenhydramine, and valerian in healthy elderly subjects. Journal of Clinical Psychopharmacology 23(3): 260-268.
- Martin JL, Hakim AD (2011) Wrist actigraphy. Chest 139(6): 1514-1527.
- Motion Watch Publications.
- Anderson GD, Elmer GW, Kantor ED, Templeton IE, Vitiello MV (2005) Pharmacokinetics of valerenic acid after administration of valerian in healthy subjects. Phytotherapy Research 19(9): 801-803.
© 2024 Jestin V Thomas. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






