- Submissions

Full Text
Advancements in Bioequivalence & Bioavailability
Comparative Bioavailability of a New Fixed Dose Combination Tablet Containing Lumacaftor/ Ivacaftor in Healthy Subjects: A Randomized, Single-Dose, 2-Way Crossover Study
Yerino Gustavo A1*, Feleder Ethel C1, Halabe Emilia K1, Giarcovich Silvia1, Tombari Dora2, Mondelo Nélida2, Díaz Liliana2, Sakson Mario2 and Roldán Emilio JA2
1FP Clinical Pharma, Argentina
2Gador Laboratories, Argentina
*Corresponding author: Gustavo Yerino, Clinical Pharma, Buenos Aires, Argentina
Submission: August 23, 2019;Published: September 13, 2019

ISSN 2640-9275 Volume2 Issue5
Abstract
Lumacaftor and ivacaftor, a CFTR modulator that comprises both a CFTR corrector and potentiator, is indicated as a fixed dose combination tablet for the treatment of cystic fibrosis in patients ≥12 years old with homozygous F508del-CFTR. The CFTR is a protein chloride channel responsible for the regulation of salt and water absorption and secretion. The purpose of this study was to compare rate and extent of absorption and to evaluate the bioequivalence between a new pharmaceutical equivalent film-coated tablet formulation containing a fixed-dose combination of lumacaftor/ivacaftor 200/125mg and the innovator product.
A single-center, open-label, randomized-sequence, single-dose, two-way, crossover bioequivalence study in 42 healthy adult subjects was performed. A wash-out period of 14 days separated dosing formulations. All subjects signed an informed consent form. Healthy male and female volunteers (non-pregnant and non-lactating) between 21-55 years with a Body Mass Index of 19-27kg/m2 were enrolled. Blood samples were collected in vacutainers containing EDTA over 72 hours.
Plasma levels of both lumacaftor and ivacaftor were measured by a validated HPLC/MS-MS method. No difference was found regarding rate and extend of absorption between products. The 90% confidence interval (CI) of the ratio of the geometric means for log-transformed Cmax, AUC0-last and AUC0-inf variables were used to determine bioequivalence between the two products using the equivalence range of 80 and 125%. Adverse events were recorded from the screening to the follow-up visit. Rate and extent of absorption were equitable between products. The point estimate and 90%CI of the ratios of Cmax, AUC0-last and AUC0-inf values for lumacaftor were 0.88(0.82-0.94), 0.92(0.86-0.99) and 0.90(0.82-0.97), respectively and for ivacaftor were 0.93(0.83-1.03), 0.96(0.87-1.05) and 0.95(0.87-1.03), respectively. Both treatments showed alike tolerability and safety. The new pharmaceutical formulation resulted bioequivalent to the innovators thus concluding that both products are therapeutically equivalent and interchangeable.
Keywords: Pharmacokinetics; Lumacaftor/ivacaftor; Fixed-dose combination; Bioequivalence; Healthy subjects
Keywords:
AE: Adverse Event; ASD: Amorphous Solid Dispersion; AUC: Area Under the Curve; BCS: Biopharmaceutical Class System; BMI: Body Mass Index; CF: Cystic Fibrosis; CFTR: CF Transmembrane Conductance Regulator; CTAB: Hexadecyltrimethylamonium Bromide; CV: Coefficient of Variation; Cmax : Maximum Concentration; FDC: Fixed-Dose Combination; FEV: Forced Respiratory Volume; FDA: Food and Drug Administration; FSH: Follicle-Stimulating Hormone; HBE: Human Bronchial Epithelial; HPMCAS: Hydroxypropyl Methylcellulose Acetate Succinate; IFC: Informed Consent Form; IVA: Ivacaftor; LUM: Lumacaftor; MOH: Ministry Of Health; PK: Pharmacokinetics; Tmax: Time To Reach The Cmax.
Introduction
Lumacaftor (LUM) has been clinically developed in combination with ivacaftor (IVA) as a fixed-dose combination (FDC) tablet for oral administration for the treatment of Cystic fibrosis (CF), a chronically autosomal recessive genetic disease affecting more than 70,000 people worldwide with a median age of death of approximately 30.6 years in the United States [1,2]. CF is caused by a mutation in the gene that encodes for the CF transmembrane conductance regulator (CFTR) protein [3]. The CFTR protein is an epithelial chloride channel located in multiple organs, being responsible for maintaining the regulation of salt and water absorption and secretion [4].
The most prevalent mutation is a deletion in the CFTR gene resulting in a loss of phenylalanine at position 508 in the CFTR protein (F508del-CFTR) that avoids the flow of chloride ions across epithelial cells leading to a cycle of mucus accumulation, infection, inflammation and progressively declining lung function [5]. Patients who are homozygous with F508del-CFTR defects have few or no CFTR protein at the cell surface and suffer from a severe form of CF disease [6]. LUM is an F508del-CFTR corrector which facilitates the cellular processing and trafficking of F508del-CFTR to raise the amount of functional CFTR protein at the cell surface, while IVA is a CFRT potentiator that increases the channel function of the CFTR protein located at the cell surface in human bronchial epithelial (HBE) cells from CF patients with homozygous F508del- CFTR [7]. The combination of LUM/IVA has higher quantity of chloride transport as compared to LUM alone in F508del/F508del- HBE cells [6,7].
Clinical efficacy and safety of the FDC of LUM/IVA in CF target population has been established in two phase III randomized controlled trials and in an extension study that demonstrated a statistically significant improvement in lung function (FEV1) that was maintained up to 48 weeks at the LUM 400mg bid/IVA 250mg bid dose regimen [8,9]. The association of LUM/IVA (CAS Nº R07AX30) in FDC (200/125mg) film-coated oral tablet was approved by the Food and Drug Administration (FDA) in 2015 for the treatment of CF in patients 12 years of age and older with homozygous F508del-CFTR being the first drug for CF directed to treat the cause of the disease [6,10].
The pharmacokinetics (PK) of LUM/IVA has been primarily obtained in healthy subject studies, considering CF is a rare disease. The PK profile was completed with sufficient data in the CF patients in phase II/III studies [6]. The exposure (AUC) of LUM is approximately 2-fold higher in healthy adult subjects compared to exposure in patients with CF. The PK of IVA, when administered with LUM, is similar between healthy adult subjects and patients with CF [1,6]. After oral dosing, both LUM and IVA show equitable systemic bioavailability. The exposure of LUM generally increased proportional to dose over the range of 50mg to 1,000mg every 24 hours and the PK of IVA showed to be linear with doses of 150mg every 12 hours to 250mg every 12 hours [1,7].
Following the proposed dose of 400mg bid, the time to reach the maximum concentration (Tmax) for LUM is 3 to 6 hours and the mean maximum concentration (Cmax) is 23,700.0ng/ml while the Tmax for IVA when administered in the FDC is approximately 4 hours and Cmax for the proposed dose of 250mg bid is 1,330.0ng/ ml [6,7]. The effect of food on the relative bioavailability of LUM/ IVA in a FDC oral tablet formulation was evaluated in a PK study in healthy subjects. Following administration of a single 400/250mg dose of LUM/IVA with a high-fat, high-calorie meal, the exposure of LUM increased approximately by 2-fold and IVA exposure raised by 3-fold when compared under fasted conditions [11].
Therefore, the FDC tablet of LUM/IVA is recommended to be administered with fatty foods [1,6]. Both LUM and IVA are 99% bound to proteins. The terminal half-life of LUM is approximately 26 hours with a clearance CL/F (CV) of 2.38L/h (29.4%) in patients with CF. When IVA was administered with LUM, the terminal halflife was approximately 9 hours in healthy subjects and CL/F was estimated to be 25.1L/h (40.5%) in patients with CF [6]. LUM is eliminated as primarily unchanged drug in the feces while IVA is extensively metabolized by CYP3A before being eliminated by the same route [7].
Both LUM and IVA are practically insoluble in water and buffer solutions with pH 1.0-8.0, simulated intestinal fluids. LUM is considered to be a biopharmaceutical class system (BCS) Class II (low solubility/high permeability) [6]. IVA belongs to BCS class II or IV. However, its low solubility and nonspecific binding to culture materials precluded an acceptable determination of its permeability using the Caco-2 cell system. Therefore, IVA could not be classified definitively [11]. Moreover, multiple polymorphic forms have been identified for LUM and IVA [6,11].
Therefore, bioequivalence studies are relevant when a generic version of the FDC tablet of LUM/IVA is manufactured. Generic drug products have been approved by the FDA supported by studies exhibiting that they are bioequivalent to the innovator products. Bioequivalence can be established on the premise of the Cmax of the drug and the AUC [12]. According to published literature, no studies regarding comparative bioavailability between a generic FDC oral tablet containing LUM/IVA and the brand-name product are available.
A new pharmaceutical equivalent immediate-release filmcoated tablet for oral administration containing an FDC of 200mg of LUM and 125mg of IVA has been developed in Argentina. A new development process for LUM synthesis allows significant savings in cost. Hence, the product can be offered with more accessible prices to countries in which the treatment is costly. The objective of the present study was to evaluate and compare the bioavailability by the rate and extent of absorption of this new generic formulation of LUM/IVA to that of the brand-name product in healthy adult volunteers under fed condition. Of interest, this is the first report on pharmacokinetics data of the combination of LUM/IVA on local population for both, the test and the reference product.
Materials and Methods
Study design and setting
A single-center, single-dose, open label, randomized-sequence, two-treatment, two-period, balanced, crossover trial in healthy adult subjects under fed condition was performed. The study was executed at the FP Clinical Pharma Pharmacokinetic Unit, Buenos Aires, Argentina, during February and April 2018 (Figure 1 summarizes the study design). All clinical operations were carried out in concordance to the Ethical Principles for Medical Research Involving Human Subjects enunciated in the latest version of the Declaration of Helsinki (2013), according to the ICH-Good Clinical Practice guidance, and to the FDA guidance for conducting bioavailability and bioequivalence studies for oral administered drugs [12-14].
Figure 1:Study design to compare the pharmacokinetics of a single dose of two FDC tablets containing LUM/ IVA formulations in healthy subjects.
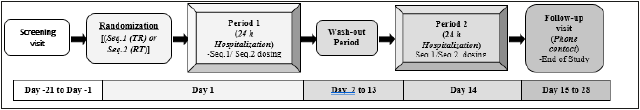
The study protocol and the Informed Consent Form (IFC) were both reviewed and approved by the Institutional Review Board, the Independent Ethic Committee (Comité de Ética en Investigacion Clinica “CEIC”, Argentina) and the local Regulatory Agency (ANMATMOH) before the initiation of the study. A written approved ICF was provided from all subjects who agreed to participate prior to the launching of the study.
Interventions
The study individuals were randomly allocated to receive the single doses of LUM/IVA, two tablets of 200/125mg (as it is the established recommended dose twice daily), each in one of two treatment sequences (Test-Reference or Reference-Test) to minimize assignment bias in compliance with the FDA guidance [15]. LUM/IVA was administered either in two FDC film-coated tablet 200/125mg of Lucaftor® as test preparation (batch No. 325550), manufactured by Gador SA Laboratories (Argentina) or in two FDC film-coated tablet 200/125mg of the innovator product Orkambi® (batch No.W034456A), manufactured by Vertex Pharmaceuticals Inc. (Boston, USA) as reference preparation which was purchased abroad. The single doses were administered with 240ml of water within 30min after a high-calorie, high-fat breakfast provided after an overnight fast of minimum 10h. The meal complied with the FDA guidance on high-fat (50% of total caloric content of the meal), high-calorie (800-1000cal) content and consisted of two eggs fried in butter, two strips of bacon, two slices of toast with butter, four ounces of hash brown potatoes, and eight ounces of whole milk [15,16].
The treatments were administered in two different dosing periods in concordance to the pre-established randomized sequence of treatment. A 14 days wash-out period between doses was included to allow for appropriate clearance of the drug and thereby avoid carryover effects from the first treatment period. Subjects were not authorized either to chew or crush the study medication and water consumption was restricted to one-h predosing and until 2h after dosing. Mouth checks were performed after dosing. Then, subjects remained under fasting condition until after the 4h pharmacokinetic blood sample time point. A standard lunch, an afternoon meal and a dinner were administered after the 4th, 8th and 12thh of drug administration, respectively. The study medication was preserved while on study following the abode conditions indicated by the prescribing information of the product provided by the sponsor.
Study population
Sample size was estimated using the formula developed by Marzo & Balant [17], considering a coefficient of variation (CV) of LUM and IVA of approximately 30% for PK parameters based on a population PK analysis reported in the literature [11]. A total of 42 healthy male and female adult subjects (non-pregnant, non-lactating) between 21 and 55 years of age with a body mass index (BMI) of 19 to 27kg/m2 were enrolled. Female subjects of childbearing potential (i.e. not surgically sterile or who have been post-menopausal for less than 2 years or menopause not confirmed by follicle-stimulating hormone [FSH]) were required to have a negative pregnancy test at screening.
Additionally, they were urged to accord to use a highly effective non-hormonal contraception method while on study treatment and during a period of 14 days after the last dose of the study drugs. Vital signs (heart rate, systolic and diastolic blood pressure, axillary temperature), laboratory tests (hematology, biochemistry, coagulation test, urinalysis) and 12-lead ECGs were required to be within normal range. Negative test for HIV, hepatitis B and C viruses were also demanded to enter into the study. Subjects were prevented from entering the study if they had a history or present evidence of gastrointestinal disease or surgery, or cardiovascular, respiratory, hepatic, renal, hematopoietic, endocrine-metabolic, neurological or psychiatric diseases. Subjects who revealed a history of alcohol or drug abuse in the last year were also excluded.
Individuals were not permitted to use any type of medicine, including herbal preparations within the previous two weeks and all through the study prosecution. Smokers were demanded to constrain from using any type of tobacco while on the study. Other standard exclusion criteria for BD/BE studies were adopted for subject enrollment [12]. Subjects were asked to refrain from foods and beverages intake with xanthines or alcohol and to avoid sun exposure, strenuous exercise and sports for 72h before the administration of the research product and during the confinement at the pharmacokinetic unit.
Sample collection and bioanalytical method
Serial blood samples for pharmacokinetic evaluations were collected by venipuncture over a 72h period at the following points: 0 (pre-dose), 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 48 & 72h after oral administration of each treatment (sample collection was stopped at 48h post-dosing for IVA analysis). A volume of approximately 10ml of blood for each sample was collected into vacutainers containing K2 EDTA as an anticoagulant. Plasma was separated by centrifugation then frozen at -20 ℃ until analysis.
An analytical method was developed and validated to determine both LUM and IVA concentrations in K2 EDTA human plasma using a high-performance liquid chromatography HPLC-MS/MS method. The method of analysis of LUM and IVA in plasma was based on an extraction with acetonitrile and sulfamethoxazol (internal standard) followed by centrifugation to segregate plasma from proteins. Then, the supernatant was injected into an isocratic system by HPLC-MSMS. Quantification of both LUM and IVA were carried out using the internal standard method. Software Lab Solutions, version 5,86 SP1, SHIMADZU Corporation was used for quantification and peak integration. A calibration curve was constructed for peak area ratios using a weighted (l/concentration2) linear least-squares regression curve method for both LUM and IVA.
The lowest limit of quantification (LLOQ) and upper limit of quantification (ULOQ) for LUM were 150.0ng/ml and 30,000.0ng/ ml, respectively. The LLOQ and ULOQ for IVA were 50.0ng/ml and 2,000.0ng/ml, respectively. The calibration curves demonstrated to be linear over the calibration concentration range (r >0.99) for each mobile phase. The accuracy and precision at the LLOQ and ULOQ of the validated method was assessed using 3 separate analytical runs, each containing 5 quality control (QC) levels for both LUM (LLOQ, LQC: 450.0ng/ml; MQC: 5,000.0ng/ml, HQC: 7,500.0ng/ ml and EXTQC (extension level): 20,000.0ng/ml) and IVA (LLOQ, LQC: 150.0ng/ml; MQC: 750.0ng/ml, HQC: 1,150.0ng/ml and EXTQC: 1,750.0ng/ml) covering the linear range of quantification in replicates of 5 performed in different days. Inter-and intra assay accuracy had mean BIAS values within±15% of the nominal values and within±20% at the LLOQ. Inter and intra assay precision had a coefficients of variations (CVs) of <15% and <20%, respectively at the LLOQ. The methodological validation was conducted following the FDA guidance for bioanalytical method validation [18].
Biopharmaceutical aspects
To avoid bio failures, special considerations were taken regarding the polymorphism of both active ingredients. In this context, attention was given to maintaining a stable form of IVA since it was a solid amorphous form, mitigating the risk of polymorphic transformation (to more stable crystalline phases) during the shelf life of the pharmaceutical product. The thermodynamically favoured conversion to lower energy and more stable crystalline forms of the amorphous forms demands to use a key strategy to overcome this challenge [19].
Therefore, IVA has been manufactured as a tertiary amorphous solid dispersion (ASD) obtained by spray drying techniques involving a stabilizing polymer HPMCAS and a surfactant for its faster dissolution [20]. HPMCAS is considered to be an unique polymer for use in ASD of poorly soluble and amorphous substances such as IVA, due to its high solubility in organic solvents and its amphiphilic characteristics that allows its interaction with hydrophobic regions of the drugs whereas the hydrophilic regions allows them to remain as stable colloids in aqueous solutions [21]. From the biopharmaceutical approach, these ASDs provide supersaturation on in vitro dissolution determinations and large bioavailability that increases the bioavailability in human studies due to more than 21 active ingredients formulated with this polymer [19,21]. The formulation of the Test product is as follows: Lumacaftor:200mg/ Ivacaftor:125mg. Excipients: Hydroxypropyl Methylcellulose acetate succinate (HPMC-AS), Povidone K25, Sodium lauryl sulfate, Croscaramellose sodium, Microcrystalline cellulose, Magnesium stearate, Alloy red lacquer 4R, Black iron oxide.
In vitro dissolution tests
In vitro comparison dissolution tests of both finished products of LUM/IVA 200/125 mg film-coated tablets (generic version) and LUM/IVA 200/125mg film-coated tablets (innovator product) containing the active principles ingredients LUM and IVA, were studied according to the dissolution conditions indicated by FDA [22]. (USP apparatus type II employing a paddle stirrer at 65rpm using as dissolution medium: 900 ml of 0.5% hexadecyl trimethyl ammonium bromide (CTAB) in acetate buffer pH 4.5, for LUM and 900ml of 0.4% sodium lauryl sulfate in phosphate buffer pH 6.8 for IVA, both at 37 ℃). Both formulations exhibited similar dissolution behavior (Figure 2A & 2B). These results are consistent with dissolution profiles for LUM and IVA reported previously in the literature [11].
Figure 2A:In vitro dissolution profiles of LUM of the test and reference FDC tablets of LUM/IVA 200/125mg. Test: blue and reference=red.
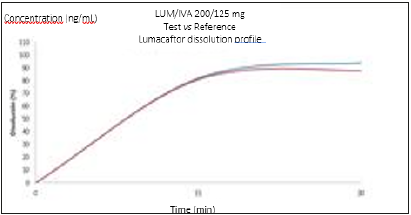
Figure 2B:In vitro dissolution profiles of IVA of the test and reference FDC tablets of LUM/IVA 200/125 mg. Test: blue and reference=red.

Pharmacokinetic evaluation
The plasma concentration-time data after oral administration of a single dose of test and reference treatment were determined by a non-compartmental pharmacokinetic model (WinNonlin, version 6.4; Certara, US). The maximum plasma concentration and the time of their occurrence were defined as Cmax and Tmax, respectively. The slope of the log-linear regression function (λ) was the first order rate constant associated with the terminal portion of the curve estimated by linear regression of time vs. log-concentration.
The trapezoidal rule was used to calculate the area under the plasma concentration-time curve from the time of dosing to the last measurable concentration (AUC0-last). The AUC from dosing time extrapolated to infinity based on the last observed concentration (AUC0-inf) was computed by the formula AUC0-inf = AUC+(Cn/λ) where Cn is the last measurable concentration and λ is the slope of the log-linear regression function. The elimination half-life (T½) was determined as ln2/λ. A pharmacokinetic rule was generated to treat data coming from samples showing values less than the lower level of quantification in bioanalytical assays. Subjects were excluded from the PK population analysis of both LUM and IVA in case of emesis occurrence at or before 2 times the median Tmax [12].
Safety assessment
Physical examination, hematology, serum chemistry (fasting glucose, urea, creatinine, liver function panel, blood clots tests), urinalysis, and a 12-lead ECG were performed at the screening visit (Day-21 to -1) for safety objectives. A urine pregnancy test was performed at screening visit and previous to each dosing period for female with childbearing potential. An abbreviated physical examination before drug administration was also performed in the morning. Vital signs measurements (heart rate, systolic and diastolic blood pressure in supine position and axillary temperature) were recorded at the screening visit, before drug administration and at time-points 2.0, 4.0, 8.0, 12 and 24h after drug administration. Adverse events (AEs) were recorded either by interrogating the subject’s general health-related questions before the dosing period and by the subject self-reporting during the study and until the follow-up visit. The seriousness, intensity (eg: mild, moderate, severe) and relationship to the study medications of the AEs were assessed by the investigators.
Statistical Analysis
The following pharmacokinetic parameters: Cmax, AUC0-last, and AUC0-inf were analyzed for LUM and IVA using natural logtransformed data. These PK variables were compared by means of ANOVA for a 2-treatment crossover design. The model covered the fixed effects of period, sequence and treatment and the random effect of subjects within sequence. The average LUM and IVA bioavailability of test formulation relative to the reference formulation was expressed as the ratio of respective estimated mean exposure and 90% confidence intervals (CIs) in terms of Cmax, AUC0-last and AUC0-inf. Schuirmann two one-sided t test was employed to compare μT/μR ratios for the PK parameters. In agreement with scientific standards and international guidelines for bioequivalence studies, bioequivalence was established if the 90% CIs for the ratio of the geometric least-squares means (test treatment/reference treatment) was within the limits of 80% to 125% for the primary PK parameters. All statistical tests used a 5% level of significance [12,23].
Result and Discusión
Subject population
A total of 42 healthy subjects were enrolled in the study. Five subjects were considered screening failures due to personal reasons and did not receive the study medication. Therefore, a total of 37 subjects were randomized to the sequence groups. At the end, 35 subjects completed the study in agreement with the protocol. Table 1 summarize the demographic characteristics of study subjects.
Table 1:Demographic characteristics of study subjects recruited for the LUM/IVA pharmacokinetic study.

Pharmacokinetics
Figure 3A:Mean plasma concentration-time curves for LUM (n=35) following single dose (400mg) administration of test and reference FDC tablets in healthy subjects under fed condition. Test=triangle and reference=circles.
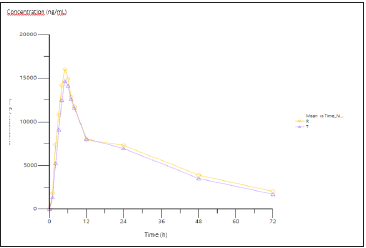
Figure 3B:Mean plasma concentration-time curves for IVA (n=35) following single dose (250mg) administration of test and reference FDC tablets in healthy subjects under fed condition. Test=triangle and reference=circles.
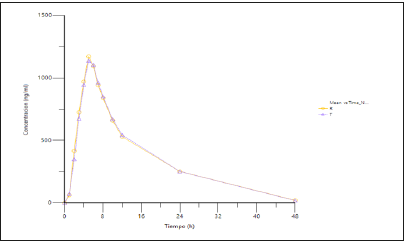
Table 2:Pharmacokinetic parameters of LUM/IVA in healthy subjects (n=35) after 400/250mg (2 x200/125mg FDC tablet) single oral dose of test or reference treatment under fed condition.
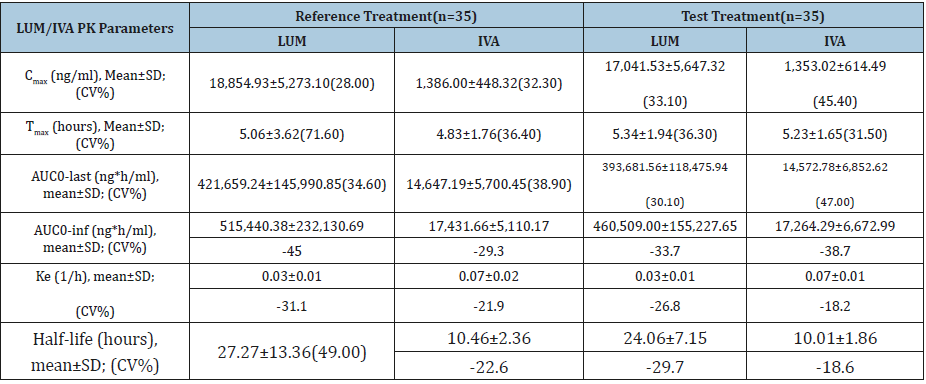
CV%=Coefficient of variation.
The PK analysis population involved 35 subjects. Figure 3 shows mean plasma concentration-time curves after single dose administration of LUM/IVA 400/250mg of test and/or reference products. The two treatment curves constituted a typical profile for a conventional immediate release formulation with long halflives and both curves were essentially comparable. After the attainment of Cmax both LUM and IVA concentrations decreased in a biphasic manner for both the test and reference products. LUM/IVA formulations exhibited almost the same mean Tmax and half-life values. Plasma pharmacokinetic parameters for LUM/IVA are summarized in Table 2. No statistical differences were shown between mean pharmacokinetic parameters for LUM/IVA test and reference formulations.
The analysis of variance did not show any statistically significant difference between the test and the reference formulations (p>0.05) in respect to the fixed effect of period, sequence, treatment and subjects within sequence as random effect for the pharmacokinetic parameters analyzed: ln Cmax, ln AUC0-last and ln AUC0-inf.
Statistical analysis of LUM and IVA pharmacokinetic logtransformed parameters and their geometric least squares mean ratios for the test and reference treatment are displayed in Tables 3 & 4. Figure 4 shows mean plasma log-concentration-time curves after single dose administration of LUM/IVA 400/250mg of test and/or reference products. The limits of the 90% CIs for the ratios of Cmax, AUC0-last, and AUC0-inf for their log-transformed data launched well within 80 to 125%. Coefficients of intra-individual variation for Cmax, AUC0-last and AUC0-inf were 0.16, 0.17 and 0.20, respectively (LUM); and 0.27, 0.24 and 0.20, respectively (IVA). Coefficients of inter-individual variation for Cmax, AUC0-last and AUC0-inf were 0.26, 0.25 & 0.31, respectively (LUM) and 0.27, 0.31 and 0.25; respectively (IVA). Test-Reference ratio for the geometric means (%) for all primary pharmacokinetic metrics (AUC0-t, AUC0- inf, Cmax) and the corresponding two-sided 90% CIs were restrained within the predefined limits of 80% to 125% (Tables 3 & 4). The null hypothesis of the two one-sided Schuirmann’s t-test could be rejected (p<0.05).
Figure 4A:Mean plasma log-concentrationtime curves for LUM (n=35) following single dose (400mg) administration of test and reference FDC tablets under fed conditions. Test=triangle and reference=circles.
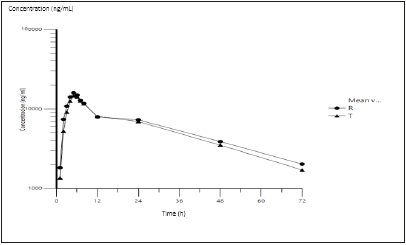
Figure 4B:Map
The purpose of this study was to assess and compare rate and extent of absorption of a new pharmaceutical oral equivalent tablet formulation containing a FDC of LUM/IVA 200/125mg to that from the innovator product in healthy subjects under fed conditions; and secondarily to evaluate bioequivalence between them. This study demonstrated that no significance differences were found, in terms of rate and extent of absorption, between test and reference products, as stipulated by Cmax and AUC comparisons and also by the similarity of plasma LUM and IVA concentration-time curves profiles. Also, the null hypothesis that the estimated parameters surpassed limits of acceptance was rejected and both formulations were found to be bioequivalent, judging that 90% CIs of the ratios of μT/μR for the PK parameters (Cmax and AUCs log-transformed) were found to be within the predetermined range (80%-125%) and the Schuirmann´s two one-sided t test procedure (probability of surpassing limits of acceptance) found all probability values <0.05.
Table 3:Bioequivalence analysis for LUM following single-oral dose administration of either test or reference drug (400 mg) to healthy subjects (n=35) under fed condition
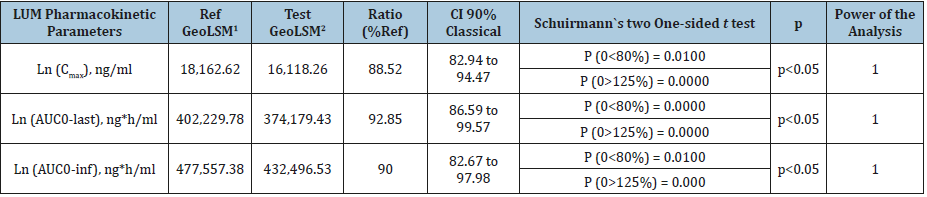
1) Ref Geo LSM= Reference Geometric Least Squares Mean;
2) Test Geo LSM= Test Geometric Least Squares Mean;
Table 4:Bioequivalence analysis for IVA following single-oral dose administration of either test or reference drug (250mg) to healthy subjects (n=35) under fed condition.

1) Ref Geo LSM= Reference Geometric Least Squares Mean;
2) Test Geo LSM= Test Geometric Least Squares Mean;
This is the first report exposing single-dose bioequivalence between a new FDC oral tablet formulation of LUM/IVA 200/125mg and the innovator FDC carried out in the same population in which the test product will be primary marketed.
Parameters of bioavailability observed in the present study are in good concordance with a previous report of a phase I, randomized, single-dose, open label, crossover study that investigated the effect of food on the relative bioavailability of two FDC of LUM and IVA tablet formulations in healthy adult subjects (n=28) which were used in the pivotal studies (VX-809-012) [11]. In this previous study, after a single oral dose of LUM/IVA 400/250mg under fed condition (high-fat, high calorie breakfast), the mean Cmax and the mean AUC0- inf for LUM were 22,400ng/ml and 565,000ng*h/ml being 2.2 and 1.6 times higher than when taken in a fasting state; and the Cmax and AUC0-inf for IVA were 1,490ng/ml and 1,8700ng*h/ml being 2.6 and 3.7 times higher than in fasting state. The median time (range) to reach the maximum concentration (Tmax) of LUM and IVA were 4.0h (2.0; 9.0) and 4.0h (2.0; 6.0), respectively in the fed state [11]. In our report mean calculated Cmax from test and reference FDC tablet for LUM (17,041.5 and 18,854.9ng/ml, respectively) were slightly lower than mean values reported in that food effect study.
These results may be partially justified due to the fairly large variability of LUM pharmacokinetic parameters, being a possible source of variability differences not clearly understood in the study population of Latin-American subjects. Likely, variations in this population are observed mostly at Tmax and AUC0-inf of LUM parameters of the reference formulation (see SD´s at Table 2). The variability (expressed as CV%) of LUM Cmax ranged from 20% to 30% over single-dose and multiple-dose studies in healthy subjects and the inter-individual CV% on clearance of LUM was reported to be 28% [6,11] The PK parameters reported in the present study were also in agreement with PK values exhibited in a multipledose study (VX-809-009) of a FDC tablet of LUM/IVA at doses of 400/250mg bid during 14 days in healthy adult subjects (n=18) including similar values for LUM and IVA Tmax. than reported in our study [11].
The pharmacokinetic parameters of LUM/IVA at steady state in patients with CF after multiple oral doses of 400/250 bid mg under fed condition during 28 days (n=56), showed a mean AUC0- 12h for LUM and IVA of 198,000ng*h/ml and 3,660.0ng*h/ml. respectively, reflecting a lower exposure values than those from test and reference formulations observed in our study. This could be explained by the fact that after twice-daily dosing, steady state plasma concentrations of LUM/IVA are generally achieved after 7 days of administration being the steady-state exposure of IVA lower than that of Day 1 due to the CYP3A induction effect of LUM [7]. Due to this metabolism interaction between the compounds, the dose of IVA for the FDC tablet (250mg orally twice daily) is augmented compared to standard monotherapy (150mg orally twice daily). Also, at steady state, it was demonstrated in previous PK studies that LUM exposure was 60% higher in healthy subjects than subjects with CF (0.875 vs 0.55), while IVA exposure was similar in healthy subjects and CF subjects [1,6].
Mean LUM/IVA half-life values for the test and reference formulations, did not diverge from previous reported data both in healthy adult subjects and in CF patients [7,24]. Recent evidence from a systematic review has demonstrated that combination therapy (LUM/IVA) results in improvements in clinical outcomes in the target population; specifically improvements in quality of life (moderate-quality evidence), in respiratory function (high-quality evidence) and lower pulmonary exacerbation rates (moderatequality evidence) [25]. LUM/IVA is actually the only oral agent in its class accessible and constitutes a highlight in the development of therapies for the treatments of CF [4]. However, more clinical data regarding lung function improvement response in long-term use is necessary specially in large populations.
Safety and tolerability
Safety and tolerability were assessed in 37 subjects who received the investigational product. In general, LUM/IVA formulations were well tolerated in all subjects. No clinically significant changes in vital signs (blood pressure, heart rate or axillary temperature) were evidenced following single oral dose of LUM/IVA 400/250mg. A total of 8 non-serious AEs was reported in 7 subjects. All AEs were considered as possibly related to the investigational product (Table 5). No serious AEs were registered. The majority of the AEs (6/8) were of mild intensity. Only one AE of moderate intensity required the use of concomitant medication. All AEs exhibited complete resolution. No unexpected AE were recorded. The safety profile observed in the present study was consistent with the AEs listed in the prescribing information of the product [6,11].
Table 5:LUM/IVA adverse events per single-dose treatment to healthy subjects (n=37).
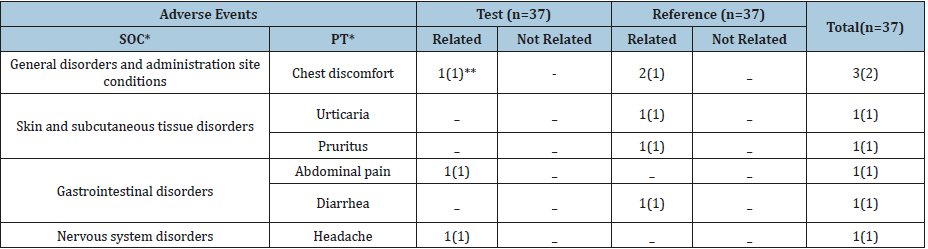
(*) SOC= System Organ Class; PT=Preferred Term, MedDRA v21.0;
(**) Number of AEs (number of subjects with AEs).
Conclusion
This study showed that both the point estimates T/R and their 90% CIs for the log-transformed Cmax, AUC0-last and AUC0-inf were in the range of 80-125%. No statistically significant difference was found for fixed effects when ANOVA test was applied to the ln Cmax, ln AUC0-last and ln AUC0-inf. Formulations were similar in terms of rate and extent of absorption. This study demonstrated that the new pharmaceutical equivalent FDC 200/125mg oral film-coated tablet formulation is also bioequivalent to the reference product. Considering the test product is pharmaceutical equivalent and bioequivalent to the reference product, then both products are judged therapeutically equivalent and therefore interchangeable.
Acknowledgment
All authors have approved the final article. All authors thanks to Gador SA Laboratories, Buenos Aires, Argentina for the financial support.
Conflict of Interest
The authors GA Yerino, EC Feleder, EK Halabe and S Giarcovih state no conflict of interest in relation to the present manuscript. The study has been financed by Gador SA. Author ER is the Scientific Director of Gador SA. Author LD is Pharmaceutical Development Manager of Gador SA. Author MS is Analytical Development Manager of Gador SA. Author NM is Research and Development Manager of Gador SA.
References
- Orkambi® (2019) (Lumacaftor/Ivacaftor) Tablets for oral use. Vertex Pharmaceuticals Inc., Boston, MA, USA.
- Cystic Fibrosis Foundation (2017) Patient registry annual data report.
- Rogan MP, Stoltz DA, Hornick DB (2011) Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation-specific treatment. Chest 139: 1480-1490.
- Bulloch MN, Hanna C, Giovane R (2017) Lumacaftor/ivacaftor, a novel agent for the treatment of cystic fibrosis patients who are homozygous for the F580del CFTR mutation. Expert Rev Clin Pharmacol 10(10): 1055-1072.
- Cant N, Pollock N, Ford RC (2014) CFTR structure and cystic fibrosis. Int J Biochem Cell Biol 52: 15-25.
- Committee for Medicinal Products for Human Use (CHMP) (2015) Lumacaftor/Ivacaftor assessment report. Procedure No. EMEA/H/C/003954/0000.
- Arends A, Pettit R (2015) Profile of lumacaftor/ivacaftor combination: potential in the treatment of cystic fibrosis. Orphan Drugs: Research and Reviews 5: 61-68.
- Brewington JJ, McPhail GL, Clancy JP (2016) Lumacaftor alone and combined with ivacaftor: preclinical and clinical trial experience of F508del CFTR correction. Expert Rev Respir Med 10(1): 5-17.
- Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, et al. (2017) Assessment of safety and efficacy of long-term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del-CFTR mutation (PROGRESS): A phase 3, extension study. Lancet Respir Med 5(2): 107-118.
- Food and Drug Administration (2015) FDA Approves Orkambi (lumacaftor/ivacaftor) for Cystic Fibrosis. Orkambi Approval History. Drugs.com
- Food and Drug Administration (2015) Center for drug evaluation and research. Clinical Pharmacology and Biopharmaceutics Review(s) Division of Pulmonary, Allergy, and Rheumatology Products Lumacaftor/Ivacaftor Oral Tablets (200/125mg). NDA206038.
- Guidance for Industry: Bioavailability and Bioequivalence Studies for Orally Administered Drug Products-General Considerations (2002) US Department of Health and Human Services, Food and drug Administration Center for Drug Evaluation and Research (CDER).
- Ethical Principles for Medical Research Involving Human Subjects (2013) World medical association declaration of Helsinki. Fortaleza, Brazil.
- Guidance for Industry: ICH E6 (R2) Good clinical practice: Integrated Addendum to ICH E6(R1) (2018) US Department of Health and Human Services, Food and drug Administration Center for Drug Evaluation and Research (CDER).
- Draft Guidance on lumacaftor/ivacaftor (2019) Food and drug Administration.
- US Department of Health and Human Services Food and Drug Administration. Center for Drug Evaluation and Research (CDER) (2002) Guidance for Industry. Food-Effect Bioavailability and Fed Bioequivalence Studies.
- Marzo A, Balant LP (1995) Bioequivalence: An updated reappraisal addressed to applications of interchangeable multi-source pharmaceutical products. Arzneim-Forsch/Drug Res 45(2): 109-115.
- US Department of Health and Human Services (2001) Food and drug Administration Center for Drug Evaluation and Research (CDER). Guidance for Industry: Bioanalytical Method Validation.
- (2018) Technologies to improve the solubility, dissolution and bioavailability of poorly soluble drugs. J Anal Pharm Res 7(1): 45-50.
- Xu L, Xin F, Robert OW, Feng Z (2017) Characterization of amorphous solid dispersions. J of Pharm Inv 48(21): 10-16
- Friesen DT, Shanker R, Crew M, Smithey DT, Curatolo WJ, et al. (2008) Hydroxypropyl methylcellulose acetate succinate-based spray dried dispersions: An overview. Mol Pharm 5(6): 1003-1019.
- (2016) FDA-Recommended Dissolution Methods: Lumacaftor; Ivacaftor. FDA Database.
- European Agency for the Evaluation of Medicinal Products (2001) Committee for Propietary Medicinal Products (CPMP): Note for Guidance on the Investigation of Bioavailability and Bioequivalence. CPMP/EWP/QWP/1401/98. London, UK.
- Fohnera AE, McDonaghc EM, Clancyd JC, Carrilloa MW, Altman RB, et al. (2017) PharmGKB summary: Ivacaftor pathway, pharmacokinetics/pharmacodynamics. Pharmacogenet Genomics 27(1): 39-42.
- Southern KW, Patel S, Sinha IP, Nevitt SJ (2018) Correctors (Specific Therapies for class II CFTR mutations) for cystic fibrosis. Cochrane Database Syst Rev, Doi: 10.1002/14651858.CD010966.pub2.
© 2018 Gustavo Yerino. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






